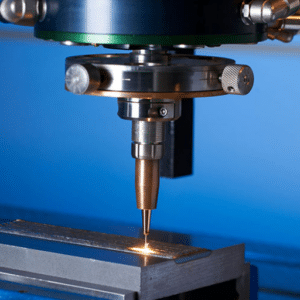
The manufacturing landscape has been disrupted by metal 3D printing, with innovative solutions that had previously seemed impossible becoming a reality. This article looks into different kinds of metal 3D printing technologies to give readers an insight into their characteristics, use cases, and advantages. As companies increasingly adopt 3D printing for complex geometries, reduced wastage, and more customization possibilities, it is essential to understand the differences among the various technologies. We will discuss Powder Bed Fusion to Direct Energy Deposition and how these methods have transformed production processes and paved the way for a brighter future in manufacturing. Whether you are experienced in this field or just entering it, this guide will take you through what matters regarding metal additive manufacturing.
What are the Main Types of Metal 3D Printing?

Image source: https://www.3dnatives.com/
Metal 3D printing technologies can be classified broadly into several main types with separate methodologies and applications.
- Powder Bed Fusion (PBF): This entails techniques such as Selective Laser Melting (SLM) or Electron Beam Melting (EBM), in which metallic powders are selectively melted layer by layer using either a laser or an electron beam leading to intricate parts.
- Direct Energy Deposition (DED): In this method, focused thermal energy such as laser or electron beams is used to melt materials as they are deposited. It is beneficial for repairing or adding material to existing components.
- Binder Jetting: This process uses a liquid binder to bond metallic powders together selectively layer-by-layer. Then, the printed articles are sintered in a furnace to achieve their final strength and properties.
- Material Extrusion: Metal filaments can also be extruded from a heated nozzle, even though polymers are most commonly utilized. This allows metal materials to be printed layer by layer, which are subsequently sintered so that they finally form into a solid state.
These methods demonstrate the variety of ways in which industries can use metal 3D printing to improve design capabilities and enhance manufacturing performance.
Understanding Metal Powder Bed Fusion
Metal Powder Bed Fusion (PBF) is a very accurate additive manufacturing process that employs a laser or electron beam to selectively melt and join metallic powders within the powder bed. The method starts with spreading a thin layer of metal powder, followed by the selective melting of regions defined by a 3D CAD model. Each subsequent layer is built on top of the previous one until completion. PBF technologies like Selective Laser Melting (SLM) and Electron Beam Melting (EBM) are prevalent in industries such as aerospace and medicine because they can manufacture complex geometries and high-strength components with minimal waste. This offers intricate designs that cannot be achieved using traditional manufacturing methods, thereby greatly expanding design freedoms and material efficiency.
Direct Energy Deposition Explained
Direct Energy Deposition (DED) is an adaptable additive manufacturing process that employs focused energy sources like lasers or electron beams to melt and deposit material in real-time. However, as in Powder Bed Fusion, DED allows the material to be deposited on existing surfaces rather than building up layers from a powder bed. In this way, the technique proves particularly efficient for repair cases where damaged parts can be reinstated without changing them altogether. In addition, DED allows for the utilization of multiple materials in one build, thus giving ample room for variability in both design and functionality. By having short build timescales and the ability to manufacture large parts with complex structures, industries can enhance their productivity and optimize materials used during production processes.
Selective Laser Melting Overview
Selective Laser Melting (SLM) is a modern additive manufacturing method in which powerful lasers combine fine metallic powders into solid objects. Such an approach involves melting the powdered material layer by layer based on a predetermined 3D CAD model, producing complex geometries with high degrees of precision. SLM has become famous for its ability to manufacture high-density parts with better mechanical properties and has, therefore, found applications in aerospace, automotive, and medical devices. Besides, this technology saves materials using only the necessary powder for each build stage. Manufacturing continues to change through SLM because it can develop personalized products and create complex designs.
How Does the Metal 3D Printing Process Work?

The metal 3D printing process typically begins with creating a digital 3D model using CAD software. The resulting model is then sliced into thin horizontal layers, and the printing commences. In Selective Laser Melting (SLM) or Direct Energy Deposition (DED), highly powered lasers or electron beams melt specific particles made from metal powders or wires according to the slices provided, and these are constructed one by one until all have been done so. After completion of every level, the build platform drops down, allowing the application of other materials on top, thus helping to develop an even more elaborate structure within impossible traditional fabrication limits. After 3D printing ends, some post-processing techniques may be applied, including heat treatment or surface finish, among others, to improve both mechanical features and the final product’s look after printing through the SLA technique, for example.
The Basics of the Sintering Process
Sintering is essential in metal 3D printing, where metal powder is made into a solid mass without fully melting. After the first printing phase, this stage aims to increase the density and mechanical stability of the printed part. During sintering, elevated temperatures are applied to the printed material, which makes particles diffuse and bond. In controlled atmospheres, more often than not, this process occurs to curtail oxidation and defect formation. If sintering is done correctly, the final product will have attained the desired strength, ductility, and other performance qualities that make it very important for use across various industries like automotive and aerospace.
Steps Involved in Metal Printing
According to my experience, metal printing has several key stages. The initial one I do is constructing a 3D CAD model, which acts as a blueprint for what my object should resemble. Following that, slicing software is used to slice up the model into layers, making it ready for additive manufacturing.
Then, I slice the design to allow me to choose suitable metal powders for loading into the 3D printer. Printing begins via Selective Laser Melting (SLM) or Direct Energy Deposition (DED), where the printer melts metal powder layer by layer based on some parts. Finally, I sinter to achieve better densities and mechanical properties for such objects after printing. Finally, post-processing may be necessary for heat treatment or surface finishing before meeting the correct specifications regarding its strength and quality upon completion of these activities. For metallic printouts, accuracy, and environmental controls are crucial factors in obtaining excellent results from this exercise during each phase.
Layer of Metal Powder in 3D Printing
In 3D metal printing, the layer of metal powder plays a crucial role in determining how well the finished product turns out. Typically, a thin film of metal powder is spread evenly across the build platform, typically at a thickness ranging from 20 to 50 microns. This specific laying flat helps control during melting and ensures each layer properly sticks to the preceding one. The quality of metal powder—size and distribution of particles—can highly impact the smoothness and density of the printed object. Moreover, powder uniformity is crucial due to defects resulting from non-uniformity, such as porosity or weak areas on final products. Effective control over the metal powder layering process is vital to achieving high-performance and reliable components in various demanding applications.
What are the Pros and Cons of Metal 3D Printing?

Metal 3D printing offers many advantages, such as its flexibility concerning design, reduced amount of waste product, and capability to make complex structures difficult for traditional manufacturing techniques. Thanks to this technology, rapid prototyping is possible, hence faster iterations and shorter production time—an advantage that puts into perspective speedups in innovation and development cycles.
Nonetheless, there are disadvantages, too. The initial costs for purchasing metal 3D printing equipment can be pretty high compared with traditional methods per part expenses through the use of this metallic printing process, mainly when producing small quantities only. Additionally, matching conventional mechanical properties often necessitates other post-processing steps, which slow down lead times and complicate workflow even more than before. Material constraints and strict environmental controls are other challenges encountered in this area’s landscape where metallic 3D printing occurs.
Benefits of Metal Additive Manufacturing
Metal additive manufacturing is preferred by many industries due to the following reasons. Firstly, it leads to a significant decrease in material wastage since parts are built layer by layer using only the amount required, which is more valuable in the case of expensive metals. Secondly, this technology makes highly complicated and intricate designs that could not have been achieved through conventional machining methods. Therefore, This gives room for innovative designs that can lead to lightweight structures and improved performance. Lastly, such rapid prototyping and customization options offered by metal additive manufacturing support accelerated development processes and quicker responses toward particular project needs or unique applications. Thus, these merits position metal additive manufacturing strongly on aerospace, automotive, and medical device manufacture, where performance and efficiency matter most.
Challenges Faced in Metal 3D Printing
I face some critical challenges in Metal Additive Manufacturing (MAM). The first one is the inconsistency of component quality, which may be due to variations in laser power or material properties that result in differences among final products made from MAM. Furthermore, during the printing process, thermal stresses could result in the warping or cracking of materials, thus complicating production processes and calling for extensive testing and validation efforts. In addition, regulatory navigation, especially in sectors like aerospace and medical devices, tends to appear intimidating when strict compliance is compulsory. Finally, there are high initial costs involved with the technology and equipment, thus becoming an obstacle for many organizations wishing to invest in 3D metal printing.
What Metal Materials Are Commonly Used in 3D Printing?

In metal 3D printing, several materials are commonly used because of their favorable properties. Stainless steel is frequently selected due to its resistance to corrosion and high strength, making it the best for different applications. Similarly, titanium alloys are popular in aerospace and medical sectors for their low weight and high strength-to-weight ratio. On the other hand, aluminum alloys have high thermal conductivity properties, but their density is relatively low, hence suitable for use with heat sinks. Lastly, nickel-based superalloys such as Inconel are highly used in harsh conditions because they can handle extreme temperatures and pressures. All these materials have specific pros that suit various industries.
Different Types of Metal Powder
In terms of metal additive manufacturing, different kinds of metallic powders are available, each with unique characteristics depending on the type of print technology.
- Stainless Steel Powder: Stainless steel powder is flexible in its uses. It doesn’t wear easily and does not corrode, making it more useful in many applications, from consumer products to industrial components.
- Titanium Powder: It is applicable in the aerospace industry due to its lightweight and high strength-to-weight ratio. Also, this material is biocompatible and thus can be employed when designing implants or other medical devices.
- Aluminum Powder: For some applications where weight vs. mechanical properties must be balanced, lightweight aluminum powder with excellent thermal conductivity would be a frequent choice. It’s a common component in the automotive and aerospace industries.
- Cobalt Chrome Powder: This material exhibits exceptional wear resistance, which makes it widely used in dental and orthopedic applications, especially when manufacturing prostheses and implants.
- Nickel-Based Superalloy Powder: Nickel-based superalloys like Inconel have been made for higher performance, primarily for environments with extreme heating and pressure, such as those found within the aerospace sector.
The choice of each type of powder depends on its particular mechanical properties and final application requirements, which ensure optimal performance across all major industrial segments.
Metal Filaments for 3D Printing
Metal filaments have become increasingly common in 3D printing. As such, their parts exhibit similar characteristics to those produced through traditional metalworking. Most of the time, these filaments comprise a combination of fine metallic powder and a polymer binder that allows them to be printed like ordinary thermoplastics. After printing, the parts go through two processes: debinding and sintering remove the binder and enable metal particles to fuse together, forming a solid metallic piece. This technique has several advantages and can help shape complicated geometries that are hard to achieve via conventional machining. Furthermore, they provide functional solid prototypes and end-use parts in industries like aerospace and automotive.
Understanding Metal Part Properties
The suitability of a material for specific applications is primarily determined by its mechanical properties. These include:
- Mechanical Properties: These include strength, ductility, toughness, and hardness. Metals like steel have high tensile strength and toughness, making them suitable for load-bearing applications. However, aluminum, which is lighter and more ductile, is preferred where corrosion resistance is needed.
- Thermal Properties: Metals’ heat conductivities differ, hence influencing their use in the aerospace or automobile industry; for instance, copper demonstrates good thermal conduction, explaining its utilization in heat exchangers, while stainless steel survives well in high temperatures due to its resistance to heat.
- Corrosion Resistance: This property denotes how long metals can withstand environmental attack from weather conditions or other factors without tarnishing. Stainless steel alloys, for example, are rust-proof and hence suitable for chemical processing ships.
By knowing these features, designers and fabricators ensure superior performance, safety, and durability by properly selecting raw materials for various projects.
What are the Applications for Metal 3D Printing?

The technology of metal 3D printing is becoming increasingly popular in different sectors due to its capability to fabricate complex shapes and customized parts. The main areas where this technology has been applied include:
- Aerospace: This industry creates light yet strong parts that enhance fuel economy and performance in air and spacecraft.
- Automotive: Production of personalized tools, prototypes, and high-performance components that improve the design and functioning of vehicles altogether.
- Medical: The creation of implants for patients, prosthetics, or instruments for surgical intervention that have enhanced fit and performance in medical procedures.
- Oil and Gas: Manufacturing durable components capable of withstanding harsh environments, thus reducing downtime and maintenance costs.
- Manufacturing: Enhancing production processes by applying metal 3D printing techniques for tooling, jigs, and fixtures that improve efficiency and accuracy.
These applications demonstrate how metal 3D printing affects various fields through innovation and improvement in operations.
Real-World Examples of 3D Printed Metal Parts
Several real-world examples have emerged as I explore the latest trends in metal 3D printing. For example, GE Aviation has successfully adopted 3D printing to manufacture fuel nozzles for their LEAP jet engines, leading to lighter components that enhance fuel efficiency and reduce production expenses. In addition, this cooperation between NASA and aerospace firms has resulted in rocket engine parts that can withstand high temperatures, demonstrating the remarkable capabilities of metal 3D printing in such environments. On the other hand, companies like Materialise manufacture bespoke metallic products such as patient-specific surgical guides and implants, proving how customization can improve accuracy and outcomes during surgery. These examples highlight how adaptable and game-changing metal 3D printing can be in any industry.
How to Choose the Right Metal 3D Printer?

We need to consider some critical elements when selecting a suitable 3d printer for metals. First, you need to look at the type of printing technology they use: Direct Metal Laser Sintering (DMLS) or Electron Beam Melting (EBM). Each comes with unique benefits depending on its applicability. Secondly, determine what is required regarding build volume and precision for your projects since these factors will dictate the part types/sizes possible to fabricate. Also, check if the machine is compatible with intended materials by ensuring it works with specific metals like titanium, stainless steel, or aluminum alloys. Lastly, evaluate the total cost of ownership: not only initial costs but also how much you will spend on maintenance, materials, and operating costs so that you choose a printer offering maximum value outlay by your organization. Considering all these factors, one can make more informed decisions aligning with the company’s production goals.
Factors to Consider in Metal 3D Printing Systems
Several key factors must be considered when evaluating metal 3D printing systems to ensure that they perform optimally and produce high-quality outputs.
- Type of Technology: Understand the differences between technologies like DMLS, EBM, or binder jetting. Each has distinct characteristics, levels of resolutions, and compatibility with materials relevant to your specific application.
- Materials: Various metals, such as titanium, stainless steel, and cobalt-chrome, can be used in 3D printing. The right system selection should allow you to choose a material for your projects while providing maximum structural strength and the desired properties.
- Post-Processing Requirements: Evaluate the inherent post-processing stages associated with each print technology. Some methods may necessitate extensive finishing procedures to achieve desired mechanical properties or surface finishes, ultimately affecting time to market and costs.
- Service Support and Maintenance: Consider the amount of maintenance each system requires and whether it has manufacturer support. By having a printer with a good customer service team and readily available spare parts, you can reduce instances of production being halted.
- Scalability and Speed: Determine if these systems are scalable in terms of production runs and the time it takes to print parts. This includes assessing build speeds and throughput, which are essential to achieving production schedules.
By examining these factors, organizations will be able to make well-informed choices about their metal 3D printing capabilities, enhancing those according to their manufacturing requirements.
Comparison of Desktop Metal Options
Many factors must be taken into account when examining Desktop Metal printers based on comments from various industrial websites.
- DMP (Digital Metal Printing) System: This system employs metal powder bed fusion technology and is best suited for high-precision applications. It is lauded for printing complicated designs with better surface finishes that suit areas like aerospace and medical.
- Studio System: This alternative can work in office environments, allowing the engineers to produce metallic parts quickly. It uses bound metal deposition, which enables complex geometries to be printed with high degrees of freedom in design. In addition, the system seamlessly integrates with post-processing solutions to improve the final output quality.
- Shop System: The shop system targets large-scale production, emphasizing speed and scalability. Its setup is very user-friendly, and operational efficiency makes it suitable for businesses needing more production without lowering their standards. Its ability to print using different materials improves its applicability across different manufacturing needs.
It also allows a company to make its 3D printing choice according to its business objectives; hence, through such a decision-making process, it gets value out of what it is buying in terms of meeting its production requirements.
Frequently Asked Questions (FAQs)
Q: What are the main types of metal 3D printing technologies?
A: The main types of metal 3D printing technologies include metal powder bed fusion 3D, metal binder jetting, and direct metal printing. Each technology utilizes different methods to fuse powdered metal materials into solid 3D-printed parts.
Q: How does metal binder jetting differ from other printing technologies?
A: Metal binder jetting is unique because it uses a binder to join layers of powdered metal rather than fusing them with heat, as in metal powder bed fusion 3D. This method allows for creating complex geometries but typically requires post-processing to achieve total density.
Q: What are the advantages of using metal 3D printing materials?
A: Metal 3D printing offers benefits such as reduced material waste, the ability to create complex and lightweight structures, and faster production times compared to traditional manufacturing technologies. Additionally, it allows for the customization of 3D-printed parts to meet specific design requirements.
Q: In what industries is metal 3D printing commonly used?
A: Metal 3D printing is used in various industries, including aerospace, automotive, medical, and tooling. These sectors benefit from the ability to produce vital, lightweight parts that can be customized for specific applications.
Q: What should I consider when designing for metal 3D printing?
A: When designing for metal 3D printing, factors such as the orientation of the part during printing, the need for support structures, and the properties of the selected metal 3D printing materials must be considered. Understanding the technology’s limitations and capabilities can help optimize the design.
Q: What is the role of a 3D printing service in metal 3D printing?
A: A 3D printing service provides expertise and equipment for producing metal parts using various metal 3D printing technologies. They can assist with the design, selection of metal materials, and the printing process, ensuring high-quality results for clients.
Q: Can you explain the process of metal powder bed fusion 3D?
A: Metal powder bed fusion 3D involves spreading a layer of powdered metal across a build platform and using a laser or electron beam to selectively fuse the powder particles. This process is repeated layer by layer until the 3D printed part is complete, resulting in a solid metal object.
Q: What is the difference between metal injection molding and 3D printing?
A: Metal injection molding is a traditional manufacturing process that combines powdered metal with a binder and then injects it into a mold. In contrast, metal 3D printing involves directly fusing powdered metal together without the need for molds, allowing for greater design flexibility and reduced lead times.
Q: What metal materials can be used in 3D metal printing?
A: Various metal materials can be used in 3D metal printing, including stainless steel, titanium, aluminum, and cobalt-chrome. The choice of material often depends on the specific application requirements and the properties desired in the final 3D printed part.