Titanium, known for its remarkable strength and lightweight properties, plays a critical role in various industrial applications, from aerospace engineering to medical implants. This article aims to delve into the fascinating world of titanium, focusing specifically on its melting point and the physical properties that make it so valuable. Through a detailed examination of titanium’s behavior under extreme temperatures, as well as a look at its common alloys, we will uncover the nuances that contribute to its widespread use. Whether you’re a materials scientist, an engineer, or simply curious about advanced materials, this blog will provide a comprehensive overview of titanium’s melting point and its influential physical characteristics.
What Is the Melting Point of Titanium?
Understanding the Melting Point of Pure Titanium
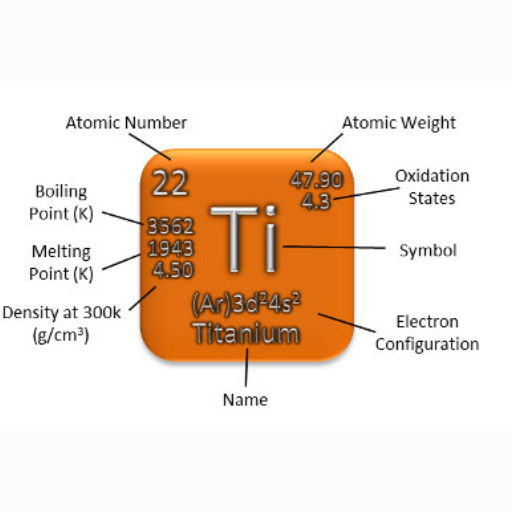
Titanium melts at about 1668 degrees Celsius (3034 degrees Fahrenheit). This relatively high temperature implies strong bonds and a crystalline structure typical for titanium metal. Consequently, understanding these basic properties is crucial to its application across industries that necessitate materials capable of withstanding extreme temperatures.
Comparing the Melting Point of Titanium to Other Metals
To put into perspective, let’s compare titanium’s melting point with that of some other commonly used metals below:
- Iron (Fe): 1,538°C (2,800°F)
- Aluminum (Al): 660°C (1,220°F)
- Copper (Cu): 1,085°C (1,985°F)
- Nickel (Ni): 1,455°C (2,651°F)
- Tungsten (W): 3,422°C (6,192°F)
Consequently titanium has a melting point of 1668 degree Celsius which is significantly higher than copper and aluminum but lower than tungsten. Due to this very factor it does have a high melting point and hence useful for high temperature applications such as jet engines and industrial furnaces. In terms of strength-to-weight ratio and corrosion resistance among others; it is one the most durable materials in different fields characterized by harsh conditions.
Factors Affecting the Melting Point of Titanium
Titanium’s melting point can vary due to a number of factors among them being purity levels, alloying elements and environmental conditions. These factors would give an in depth understanding on how titanium behaves under various given situations.
Purity: The presence of impurities can have significant impacts on the melting point of titanium. High-purity titanium has melting points that are close to its theoretical value around 1668 degrees Celsius. Nevertheless even minute quantities can lower it making composition control crucial in processing.
Alloying Elements: Also alloys formed when other metals are added will affect the melting temperature of titanium. For example:
- Aluminum (Al): Often added into titanium alloys, aluminum helps in increasing the overall melting point and strengthening such alloys.
- Vanadium (V): It can lower or maintain the melting point depending on its concentration which is usually used to improve weldability and formability in titanium alloys.
- Molybdenum (Mo): It improves creep resistance at high temperatures because it raises the melting point.
Environmental Conditions: Melting points of titanium may also be influenced by other factors including pressure and atmosphere. For instance:
- Pressure: The higher the pressure, the higher will be the melting point; lowering it would require reducing pressure.
- Atmosphere: Inert atmospheres like argon or vacuum conditions helps keep a constant melting point while reactive environments such as oxygen rich atmospheres results into oxidation hence effective drop in melting temperature.
From this, we can say that proper control of composition and environmental conditions allow for optimization of titanium performance for specific applications.
How Does the Alloy Composition Affect Melting Point?
Common Alloys and Their Melting Points
Titanium-Aluminum-Vanadium (Ti-6Al-4V)
This is among the most common types of titanium alloys. Its melting point stretches from 2,912 to 3,038 degrees Fahrenheit or approximately 1,600 to 1,670 degrees Celsius. It has added vanadium and aluminum that make it have high strength, able to resist corrosion and thermal stability making it useful in aerospace applications or even medical purposes.
Titanium-Aluminum (Ti-Al)
The titanium-aluminum alloy is also a popular alloy used especially for high temperature applications nowadays. Typically these alloys have a melting point range between 2,552 and 3,002 degrees Fahrenheit or about 1,400 -1650 centigrade. Aluminum inclusion increases hardness while maintaining its relatively high melting temperature thus making it ideal for manufacturing turbine blades alongside other highly stressed components.
Titanium-Nickel (Ti-Ni or Nitinol)
The titanium-nickel alloy has become famous for its shape memory and super elastic properties. This specific kind of alloy usually melts at temperatures ranging between 2,390 – 2399°F {1310 –1315°C}. Based on the nickel content that brings about this lower melting point compared with other titanium alloys; but this is unique in that it makes medical equipment like stents and orthodontic wires last long.
The Role of Alloying Elements in Titanium Alloys
There are several different elements that can be combined with titanium in order to create an alloy with improved properties. For instance aluminum is normally added in order to strengthen the substance whereas keeping its melting point above normal levels which makes it suitable for such uses as high temperature applications. Vanadium enhances resistance towards corrosion as well as increasing thermal stability hence commonly applied within aerospace industry plus medicine sector respectively. In addition Nickel being often used in form of Nitinol (Ti-Ni) imparts special properties such as superelasticity and shape memory in medical devices. Further, elements like chromium, molybdenum and zirconium can be added for welding improvement, corrosion resistance boosting or even strength rising respectively in titanium alloys. It is therefore evident that the smart choice of these alloying elements and their combination makes it possible for titanium alloys to suit various needs across different advanced industries.
Manufacturing Processes and Titanium Alloys
Titanium alloy manufacturing processes are complex and include several steps that ensure the desired properties in the final product. Usually, the initial stage involves extraction of titanium from its ores such as rutile or ilmenite through Kroll process which results into a sponge-like titanium. This sponge is usually melted mostly by using vacuum arc remelting (VAR) furnace thereby leading to more refined titanium ingot.
After making the ingot, various thermomechanical processes like forging, rolling or extrusion would be implemented so that an alloy might have been given required shape along with better mechanical properties. For instance, precision applications may involve techniques such as isothermal forging that ensures uniform properties throughout the component.
In latter stages; annealing, solution treating and aging (STA) among other treatments may be done on titanium alloys so as to further modify microstructure or mechanical properties suitable for certain applications depending on their specifications. Additive manufacturing technique especially 3D printing has also come up as one of promising methods particularly when it comes to production of complex geometries without wasting much material.
For example ultrasonic testing and X-ray diffraction (XRD), quality control measures are very important during manufacture of titanium alloy products most especially those used in aerospace industry plus medicine field. These comprehensive processes ensure that highly versatile materials like those made from Ti-6Al-4V reach tough standards necessary for difficult environments including those found in military applications.
Why Is Titanium Known for Its High Melting Point?
Titanium Atomic Structure
The atomic structure of titanium is known for its high melting point. Its metal form has a hexagonal close-packed (HCP) crystal structure at room temperature and changes to body-centered cubic (BCC) structure at high temperatures, around 882°C (1620°F). The strong metallic bonds maintained within these structures are responsible for its high thermal stability. Moreover, titanium atoms have large size and high binding energy levels that require more energy in order to break the bondings or melt the metal itself. These structural factors make it retain integrity under extreme temperature conditions, thereby making it suitable for use in demanding environments such as aerospace applications and industrial machinery.
The Role of Ti-Atoms in Titanium’s Properties
Titanium atoms play an important role when it comes to defining characteristics in this metal. They create a strong metallic lattice due to their large size and high binding energy which accounts for titanium’s excellent strength and durability. The arrangement of Ti-atoms within the hexagonal close-packed (HCP) and body-centered cubic (BCC) structures enhances its ability to withstand deformation and maintain stability under extreme conditions. Similarly, ti-atoms tend to have a higher affinity for oxygen, thus leading to the formation of a protective oxide layer on titanium surfaces hence increased corrosion resistance. All these factors contribute in giving titanium its extremely high melting point among other properties like biocompatibility that makes it be one of the most valuable materials used in aerospace industry, medicine as well as various manufacturing sectors.
Comparisons with Tungsten and Nickel
To compare titanium with tungsten and nickel we must look into several technical parameters including density, melting point, corrosion resistance, specific applications etc.
Density And Weight:
- Titanium: Approximately 4.5 g/cm³
- Tungsten: Approximately 19.3 g/cm³
- Nickel: Approximately 8.9 g/cm³
Titanium has a relatively lower density making it much lighter than both tungsten and nickel; a situation that is beneficial in aerospace where weight reduction is crucial.
Melting Point:
- Titanium: 1,668°C (3,034°F)
- Tungsten: 3,422°C (6,192°F)
- Nickel: 1,455°C (2,651°F)
Among these three elements tungsten has the highest melting point which makes it suitable for use in high-temperature applications such as filaments in light bulbs or aerospace components. Despite this titanium still possesses a better value of melting point than nickel thus highly regarded for its tough heat resistance and used in high-temperature alloys.
Corrosion Resistance:
- Titanium: Excellent because of protective oxide layer formation
- Tungsten: Generally good although environmental conditions may influence performance
- Nickel: Good especially when alloyed to form stainless steel and other corrosion-resistant materials
When exposed to corrosive environments for extended periods, titanium demonstrates outstanding corrosion resistance unlike all others that is ideal for medical implants and marine applications.
Applications:
- Titanium: Aerospace, medical implants, chemical processing equipment, marine applications.
- Tungsten: High temperature environments, electrical contacts and radiation shielding.
- Nickel: Stainless steel, superalloys, batteries and electroplating.
Therefore titanium best encompasses low density while its melting point is also high besides having excellent corrosion resistance which enables it to achieve wide usage across different demanding applications. Tungsten is highly resistant to temperature variations hence suited as a material for use under extremely hot conditions whereas nickel’s balanced properties combined with alloying capabilities make it suitable for numerous industrial uses.
What Are the Applications of Titanium?
The Role of Titanium in Aerospace
Titanium is a valuable material for aerospace industry due to its exceptional strength-to-weight ratio, corrosion resistance and ability to withstand extreme temperatures. Major applications are making such critical parts as airframes, jet engines, landing gears and hydraulic systems. In addition, titanium alloys are widely used to produce turbine blades, compressor discs and other high-stress parts because they are strong enough and have resistance to fatigue and crack propagation. Moreover, the low density of the material helps decrease the mass of aircrafts, facilitating better fuel consumption rate and performance. The extensive application of titanium in both civilian and military aviation attests to its significance in advancing aerospace technology while ensuring safety and dependability under challenging operational conditions.
Medical Uses of Titanium
There is wide spread use of titanium in medical field due to its biocompatibility, strength and corrosion resistance. This attribute makes it a preferred choice for orthopedic implants that include hip replacements knee replacements spinal fusion devices bone plates among others because it can integrate well with human bones. As a non-reactive substance there is minimal risk associated with rejection or adverse reaction when it comes long-term implants made from this substance Furthermore, excellent strength combined with lightness allows for fabrication of enduring yet lightweight surgical instruments as well as medical devices.In dental implantation also; titanium is very essential since it offers a stable way out which stays longer in place.Titanium has proven successful in healthcare having been adopted by many which demonstrates just how important this material has become towards enhancing healthy lives as well as positive results on patients.
Industrial Applications for Titanium
The versatility of titanium makes it useful even outside the aerospace industry including medical uses thereby becoming highly important to many sectors in industrial applications within economy. Notably, one major application area is chemical processing where equipment such as heat exchangers reactors piping systems etc call for high corrosion resistance therefore being ideal candidates for use with metals like titanium.Additionally, desalination plants are made from titanium as it cannot be corroded by seawater causing them to last longer.
The power generation industry is one of the consumers of titanium, more so in the production of turbines and exhaust systems where its high strength-to-weight ratio and ability to withstand high temperatures are necessary. What is more, titanium is currently being adopted by automotive sector which aids in making lighter vehicles that burn less fuel yet their strength and durability remain unaffected by this change.It can also be used in sports equipment including bicycles and golf clubs because of its material versatility which improves performance across different products.
How Is Titanium Produced and Fabricated?
The Metallurgy of Titanium
Having done extensive studies on titanium production, I can say that the process begins by extracting titanium ore, mostly in the form of rutile or ilmenite. The ore is then subjected to a purification process that typically employs the Kroll Process. This approach involves closeting titanium tetrachloride (TiCl4) with magnesium under an inert atmosphere to make titanium sponge. The sponge is again melted in a vacuum arc furnace thereby making ingots that can be further processed into plates, bars or other similar forms.
Other methods involve forging, rolling and adding unique features such as additive manufacturing that enable intricate shapes and designs to be achieved. In these processes, temperature and atmosphere must be well controlled for purity and properties of the material to be maintained throughout fabrication process. Every stage within the production and fabrication chain has a significant role to play in ensuring that final products meet their diverse applications requirements.
Processes Used in Reducing Titanium Tetrachloride
Regarding decrease of titanium tetrachloride (TiCl4), there are various ways which are used but most commonly known is Kroll Process. It involves placing TiCl4 inside a reaction vessel containing molten magnesium at elevated temperature usually around 800-900 degrees Celsius. High temperatures often result from argon gas being used as an inert atmosphere so as not to cause any unwanted reactions. When TiCl4 reacts chemically with Mg it produces Ti sponge plus Mg chloride (MgCl2). After washing away impurities, this metallic sponge is then purified and subsequently melted to form ingots.
Another method although less common one is Hunter Process where instead of employing magnesium sodium is used for reducing TiCl4. Sodium when mixed together with TiCl4 is placed into steel bomb reactor at about 400-800 degrees Celsius for producing titanium plus sodium chloride (NaCl). However, its scalability is typically more challenging than that offered by the Kroll Process, hence less economic. All these ways require exacting control of various parameters to yield high purity titanium which is ideal for many different applications.
Challenges in the Fabrication of Titanium Alloys
There are several major challenges associated with fabricating titanium alloys due to their distinctive properties. Firstly, high melting point and excellent corrosion resistance of titanium make it necessary to create specialized equipment capable of working at very high temperatures as well as maintaining an inert atmosphere thereby preventing oxidation. Additionally, alloying is a complicated process which necessitates accurate control over composition and cooling rates in order to achieve specific mechanical properties and microstructure. Finally, machining titanium alloys is difficult because they possess high strength but low thermal conductivity leading to fast tool wearing out and increased cost. Overcoming these issues is essential for exploiting performance advantages imparted by titanium alloys in aerospace, medical and industrial settings.
Frequently Asked Questions (FAQs)
Q:What temperature does titanium melt?
A: Titanium’s melting point is roughly 1,668° Celsius (3,034° Fahrenheit).
Q: Describe the properties of titanium that make it unique.
A: Some of the features that make it unique include excellent tensile strength, resistance to corrosion and a small mass making it light yet strong. Moreover, being biocompatible titanium is used in medical implants.
Q: How does the melting point of titanium compare to the melting point of steel?
A: The boiling point for titanium approximately is 1,668 degrees Celsius while that for steel ranges between 1,370 and 1,540 degrees Celsius.
Q: Why do aerospace applications prefer titanium metal?
A: Titanium metal is preferred in aerospace applications because it has high tensile strength but low density. It is therefore possible to develop a powerful material which can endure tough conditions without weighing a lot.
Q: Who discovered titanium and when?
A: In 1795 Martin Heinrich Klaproth discovered Titanium; he named this metal after the Titans in Greek mythology.
Q: What are some common applications for titanium and its alloys?
A: Due to their tensility, lightweight nature as well as their property against corrosive elements; titanium and its alloys are commonly used in various fields such as aerospace components, medical implants, military equipment and sports equipment.