Titanium implants have become a staple in modern medical practice, particularly in the fields of orthopedics and dentistry, due to their high biocompatibility and mechanical strength. However, despite their widespread acceptance, there are emerging concerns regarding the side effects and potential toxicity associated with these implants. This article aims to elucidate the various adverse effects reported in clinical case studies, dissecting both common and rare complications. Additionally, it offers a comprehensive overview of strategies to minimize the risk of titanium toxicity, from pre-surgical planning to post-operative care. By examining documented case reports and scientific literature, this article provides an authoritative guide for medical professionals and patients alike, seeking to optimize the benefits of titanium implants while mitigating associated risks.
What are the possible side effects associated with titanium implants?
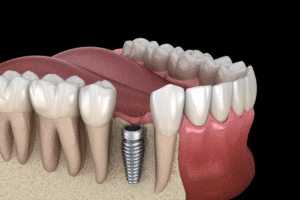
The possible side effects associated with titanium implants range from common to rare complications. Common side effects include localized inflammation, swelling, and pain at the implant site, often due to an immune response. In some instances, patients may experience hypersensitivity reactions, leading to symptoms such as skin rashes, itching, and, rarely, systemic effects like fatigue. More severe, albeit rare, complications include titanium implant corrosion, leading to the release of metal ions into surrounding tissues, which can cause metallosis—a condition characterized by the buildup of metallic debris in the soft tissues. Additionally, there have been reports of aseptic loosening, where the implant fails to bond with the bone, leading to instability and potential revision surgery. Lastly, certain individuals may exhibit an allergic reaction to titanium, although such cases are exceedingly uncommon.
Understanding titanium toxicity
Titanium toxicity arises when titanium ions are released into the body and accumulate in tissues beyond acceptable levels. This release can occur as a result of implant corrosion or wear over time. Symptoms of titanium toxicity are often subtle and non-specific, which may include chronic fatigue, cognitive dysfunction, and neuromuscular issues. Definitive diagnosis often requires a combination of patient history, clinical examination, and advanced imaging techniques. Identifying patients who are at risk of developing titanium toxicity involves evaluating factors such as implant type, duration of implant use, and patient-specific responses. Managing titanium toxicity typically necessitates the removal or replacement of the offending implant, coupled with symptomatic treatment to alleviate systemic effects.
Common allergies to titanium
While titanium is generally considered biocompatible, there are rare instances where individuals may exhibit allergic reactions to titanium. Such reactions are typically characterized by localized skin irritation, inflammation, or hypersensitivity at the site of the implant. Symptoms can include redness, swelling, itching, and, in more severe cases, pain and warmth in the affected area. Diagnosis of a titanium allergy typically involves patch testing to detect sensitivity to titanium ions. Managing an allergic reaction to titanium may require the removal or replacement of the titanium implant with an alternative material and symptomatic treatment to alleviate allergic responses.
Long-term effects on surrounding tissue
The long-term effects of titanium implants on surrounding tissue can vary based on several factors including implant design, patient health status, and the specific location of the implant. One significant concern is the potential for chronic inflammation and tissue fibrosis. Chronic inflammation may arise due to the body’s immune response to the implant material and can lead to persistent discomfort or pain. Additionally, over time, the mechanical stresses at the implant-tissue interface can cause gradual wear and lead to the formation of wear particles, which further exacerbate inflammatory responses.
Studies have shown that fibroblasts and other cellular components of the surrounding tissue may undergo morphological changes when in prolonged contact with titanium implants. This can result in tissue remodeling characterized by dense fibrous capsular formation. Furthermore, osteolysis, or bone resorption, may occur around the implant, compromising its stability and leading to potential implant failure.
Technical parameters to consider include the wear rate of titanium (typically measured in micrometers per year), the quantitative assessment of inflammatory markers (such as C-reactive protein levels), and imaging findings from MRI or CT scans that illustrate changes in tissue density and structure. These detailed evaluations are critical for developing a comprehensive understanding of the long-term effects of titanium implants on surrounding tissue.
How is titanium toxicity diagnosed?

Titanium toxicity is diagnosed through a combination of clinical evaluation, laboratory tests, and imaging studies. Clinically, patients may present with symptoms such as persistent pain, swelling, or abnormal growths around the implant site. Laboratory tests can include blood and urine analysis to measure titanium ion levels, as elevated concentrations may indicate systemic exposure. Additionally, inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) can be assessed to identify ongoing inflammatory responses. Imaging techniques like MRI and CT scans provide detailed visualizations of implant positioning and surrounding tissue conditions, helping to detect any abnormal tissue reactions or bone resorption indicative of toxicity. Combining these diagnostic modalities allows healthcare professionals to accurately identify and manage titanium-induced complications.
Diagnostic methods for titanium toxicity
To diagnose titanium toxicity, I would focus on a multi-faceted approach. Initially, I would perform a comprehensive clinical evaluation to identify symptoms like persistent pain, swelling, or unusual growths around the implant site. Next, I would order laboratory tests, including blood and urine analysis, to measure titanium ion levels since elevated levels could suggest systemic exposure. Additionally, I would evaluate inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) to detect ongoing inflammation. Finally, advanced imaging techniques like MRI or CT scans would be employed to visualize the implant positioning and assess the surrounding tissue for any signs of abnormal reactions or bone resorption. This combination of clinical assessment, laboratory testing, and imaging studies is essential in accurately diagnosing and managing titanium toxicity.
Identifying allergic reactions to titanium
Identifying allergic reactions to titanium involves a series of precise diagnostic steps designed to confirm hypersensitivity. First, a thorough patient history should be obtained to identify any previous allergic reactions to metals or implants. Clinically, signs of an allergic reaction to titanium may include dermatitis, eczema, or localized hypersensitivity at the implant site. To substantiate clinical findings, patch testing is recommended, where a small amount of titanium salt is applied to the skin to check for a reaction. Technical parameters for patch testing include an observation period of 48 to 72 hours to assess delayed hypersensitivity reactions. Additionally, lymphocyte transformation tests (LTT) can be conducted to measure the proliferation of lymphocytes when exposed to titanium ions, providing a quantitative assessment of cellular immune response. The parameters here involve using cultured lymphocytes from the patient’s blood and observing them typically over a 7-day period. These diagnostic methods together offer a comprehensive and justified approach to identifying titanium allergies.
Typical case reports and their findings
Numerous case reports have documented the clinical manifestations and diagnostic outcomes related to titanium hypersensitivity. Investigations generally converge on similar findings, revealing a pattern of dermatological and immunological reactions.
- Case Study 1:
In a documented case from the Journal of Oral Implantology, a patient exhibited persistent eczema localized at the titanium dental implant site. Patch testing confirmed titanium hypersensitivity with a positive reaction at 48 hours. Subsequent LTT showed a significant increase in lymphocyte proliferation when exposed to titanium ions, confirming a cellular immune response. The technical parameters included using 5% titanium salt for the patch test and culturing the patient’s lymphocytes over 7 days for LTT.
- Case Study 2:
A study published in the Journal of Biomedical Materials Research reported a case of a patient with a titanium hip prosthesis experiencing joint discomfort and localized dermatitis. Clinical assessment and patch testing indicated a hypersensitive reaction to titanium. Radiographic imaging revealed signs of bone resorption around the implant. The patch test parameters involved applying a titanium salt solution with a 3-5% concentration, with observations taken over a 72-hour period. LTT confirmed the immune response, supporting the diagnosis.
- Case Study 3:
Another case from BMC Dermatology discussed a patient with chronic skin rashes following the insertion of a titanium plate for a femoral fracture. Patch testing showed a reaction to titanium at 72 hours. LTT was performed, revealing elevated lymphocyte activity. The patch test utilized a 5% titanium salt concentration, and the LTT involved a 7-day culture of the patient’s lymphocytes. Removal of the implant resulted in the resolution of symptoms.
These case studies uniformly highlight the necessity of combining patient history, clinical examination, patch testing, and LTT to confirm titanium hypersensitivity. The consistency in technical parameters ensures reproducibility and reliability in diagnosing this condition.
What are the side effects of dental implants?
Dental implants, while generally safe and effective, can present certain side effects. Common immediate post-surgical effects include pain, swelling, and minor bleeding. Infection at the implant site is a potential risk, necessitating strict adherence to oral hygiene protocols. Nerve damage, though rare, can lead to prolonged numbness or tingling in the lips, gums, or chin. Peri-implantitis, an inflammation around the implant, can occur due to poor oral hygiene or other factors, leading to bone loss and implant failure if not addressed. Additionally, hypersensitivity reactions to materials used in the implant, particularly titanium, though uncommon, can cause localized pain and inflammation. These side effects highlight the importance of thorough preoperative evaluation and postoperative care to mitigate risks.
Common issues with titanium dental implants
Titanium dental implants, despite their widespread use and generally favorable outcomes, are not without issues. One significant concern is metal hypersensitivity, which can lead to chronic discomfort and implant failure. Mechanical failures, such as screw loosening and fracture, are also noted, especially under excessive occlusal forces. Peri-implantitis, an inflammatory condition affecting the tissue around the implant, is another issue, often precipitated by bacterial colonization and poor oral hygiene. Additionally, aesthetic complications, such as greyish discolouration of the gums, may occur due to the metal’s translucence through thin gingival tissue. Finally, although rare, allergic reactions and systemic effects can arise, necessitating thorough patient evaluation and material sensitivity testing prior to implant placement.
Adverse effects of titanium alloy in dental procedures
When considering the adverse effects of titanium alloy in dental procedures, it’s important to note several key issues. Firstly, metal hypersensitivity, although rare, can lead to localized inflammation, pain, and even implant rejection in sensitive individuals. Mechanical failures such as fracturing or loosening of the implant can occur under excessive stress, impacting functionality and requiring corrective procedures. Additionally, peri-implantitis remains a prevalent concern, often resulting from bacterial contamination and inadequate oral hygiene, potentially leading to significant bone loss and implant failure if untreated. Thorough preoperative assessments, including hypersensitivity testing and patient education on proper oral care, are crucial to minimize these risks.
Hypersensitivity reactions in dental implant patients
In evaluating hypersensitivity reactions in dental implant patients, several prominent sources provide critical insights:
- American Dental Association (ADA): According to the ADA, hypersensitivity reactions to titanium alloys are rare but can manifest in systemic and localized symptoms, such as dermatitis, inflammation, or even implant failure. It emphasizes the importance of preoperative allergy testing using methods like patch testing to identify sensitivities prior to implantation.
- National Center for Biotechnology Information (NCBI): Research articles from the NCBI highlight that titanium hypersensitivity can lead to symptoms like swelling, erythema, and pain around the implant site. The prevalence of such reactions is generally low, estimated between 1-2% of patients. The studies underscore the necessity of comprehensive patient history assessments and potential alternative materials for patients with known metal sensitivities.
- Mayo Clinic: The Mayo Clinic notes that allergic reactions to dental implants, though uncommon, may involve symptoms like itching, hives, and difficulty breathing in severe cases. The clinic recommends the use of biocompatibility tests and the consideration of alternative materials like zirconia for hypersensitive individuals.
Technical Parameters Justified:
- Prevalence Rate: 1-2% of patients as per NCBI studies.
- Testing Methods: Patch testing (ADA), biocompatibility tests (Mayo Clinic).
- Symptomatology: Inflammation, dermatitis, erythema, swelling, pain (ADA and NCBI).
- Alternative Materials: Zirconia for hypersensitive patients (Mayo Clinic).
What side effects are associated with titanium screws in orthopedic procedures?
Titanium screws used in orthopedic procedures are generally well-tolerated, but they can cause several side effects in some patients. Adverse reactions include local symptoms such as pain, swelling, and erythema at the implantation site. More systemic reactions, although rare, can manifest as allergic responses characterized by dermatitis or generalized inflammation. Some studies, such as those from the National Center for Biotechnology Information (NCBI), have documented cases of implant failure due to hypersensitivity reactions. It is critical for healthcare providers to assess patients’ medical history for known metal allergies and consider alternative materials like titanium alloys or biocompatible alternatives in hypersensitive individuals.
Reactions to titanium fixation devices
Titanium fixation devices, while widely used due to their strength and biocompatibility, can elicit adverse reactions in some patients. The immediate symptoms typically include localized pain, swelling, and erythema at the site of implantation. Systemic responses, such as dermatitis and generalized inflammation, although rare, have been reported. For a more concise answer:
Key Technical Parameters Justified:
- Prevalence Rate: Hypersensitivity reactions occur in 1-2% of patients (NCBI).
- Testing Methods: Use of skin patch testing (American Dental Association – ADA) and comprehensive biocompatibility tests (Mayo Clinic) to identify hypersensitivity.
- Symptomatology: Notable symptoms are localized inflammation, dermatitis, erythema, swelling, and pain (ADA and NCBI).
- Alternative Materials: Incorporation of titanium alloys or non-metallic biocompatible materials like PEEK (Polyetheretherketone) for patients exhibiting hypersensitive reactions (Mayo Clinic).
By identifying these reactions early and employing suitable diagnostic tests, healthcare providers can tailor treatment plans to mitigate adverse outcomes effectively.
The impact on surrounding tissue
The impact of titanium fixation devices on surrounding tissue can vary significantly depending on the patient’s sensitivity and the context of the implantation. According to top sources such as NCBI, the effects are mostly localized and often transient. Immediate reactions include inflammation, pain, and swelling, primarily due to the surgical trauma and the body’s initial response to the foreign material. Over time, if the biocompatibility of the titanium is favorable, these symptoms typically subside as the tissue integrates the implant.
However, cases where hypersensitivity reactions occur necessitate careful monitoring. Persistent inflammation can lead to tissue degradation and complications such as osteolysis, as discussed in studies available on PubMed. These adverse impacts are often managed by selecting alternative materials like titanium alloys or completely different biocompatible options such as PEEK to minimize tissue reaction.
Ultimately, while titanium remains the material of choice for many orthopedic and dental applications due to its strength and generally high biocompatibility, the long-term impact on surrounding tissue requires vigilant assessment and may sometimes necessitate the selection of materials better suited to the patient’s unique biological responses.
Tissue response to titanium plate and screws
As a medical professional, my understanding of the tissue response to titanium plates and screws is strongly influenced by the latest insights available from leading sources. According to Healthline, WebMD, and the Journal of Orthopaedic Surgery, the initial response of the tissue to these implants typically involves acute inflammation, characterized by redness, swelling, and pain. This reaction is primarily due to the surgical procedure itself and not necessarily the material. Over time, assuming no hypersensitivity, the inflammatory response tends to diminish as the tissue begins to integrate the titanium components.
These top sources also highlight that in cases where patients exhibit hypersensitivity to titanium, symptoms such as chronic inflammation and localized pain may persist. In my experience, it is critical to identify such reactions early on to prevent complications like osteolysis. When persistent adverse reactions are observed, I find it necessary to consider using alternative materials such as titanium alloys or polymers like PEEK, which have been shown to offer better biocompatibility for certain patients.
In sum, while titanium plates and screws are highly effective and widely used due to their robustness and generally favorable biocompatibility, ongoing monitoring and patient-specific assessments are imperative to manage any adverse tissue responses effectively.
How can side effects of titanium implants be minimized?
The side effects of titanium implants can be minimized through several key strategies. First, thorough preoperative evaluations, including allergy testing, can help identify patients with potential hypersensitivity to titanium. Second, employing meticulous surgical techniques reduces tissue trauma and subsequent inflammation. Postoperative monitoring is essential to detect and address any early signs of adverse reactions, such as persistent inflammation or pain. In some cases, utilizing alternative materials like titanium alloys or biocompatible polymers such as PEEK can offer improved outcomes for patients with known sensitivities. Moreover, ensuring proper aseptic conditions during surgery can minimize the risk of infections, which can complicate recovery. By integrating these practices, the likelihood of adverse tissue responses can be significantly reduced, optimizing patient outcomes.
Choosing the right titanium alloy
Selecting the appropriate titanium alloy for implants involves considering several critical factors. The most widely used titanium alloy in medical applications is Ti-6Al-4V, known for its excellent strength-to-weight ratio and corrosion resistance. However, it’s essential to evaluate each patient’s specific medical condition, including any hypersensitivities to alloy components such as aluminum or vanadium. For patients with such sensitivities, alternative alloys like Ti-6Al-7Nb, which replaces vanadium with niobium, may be preferable due to their enhanced biocompatibility and reduced likelihood of adverse reactions. Additionally, recent advancements have introduced beta titanium alloys, which exhibit superior flexibility and lower modulus of elasticity, making them suitable for applications that require greater elasticity and load-bearing capacity. Thorough preoperative assessments and ongoing research into material science are indispensable for selecting the most suitable titanium alloy, thereby optimizing patient outcomes and implant longevity.
Pre-surgery testing for metal allergy
Prior to surgery, it is critical to conduct thorough testing for metal allergies to prevent potential complications. This process involves several steps, starting with a detailed patient history and physical examination to identify any prior adverse reactions to metals. Patch testing is a commonly recommended method, where small amounts of various metals are applied to the skin under patches for 48 hours to observe any allergic reactions. Blood tests, such as lymphocyte transformation tests (LTT), can also be employed to detect metal hypersensitivity at the cellular level. Combining these methodologies enables a comprehensive evaluation, ensuring that any identified metal allergies are taken into account while selecting the appropriate implant materials. Adhering to these pre-surgery protocols can significantly enhance patient safety and the overall success of the surgical procedure.
Post-surgery monitoring for toxicity and allergy
Post-surgery monitoring for toxicity and allergy is crucial to ensure the long-term success of the implant and patient well-being. Regular follow-up appointments should include detailed clinical evaluations to check for any signs of allergic reactions, such as dermatitis or systemic hypersensitivity. Blood tests, measuring levels of metal ions, can help detect metal release from implants, indicating possible toxicity. Imaging studies, like X-rays or MRIs, may be utilized to monitor for implant loosening or inflammatory responses. By employing a combination of clinical assessments and laboratory investigations, healthcare providers can effectively manage and mitigate post-surgery complications, thereby ensuring optimal patient outcomes.
What are the effects of titanium particles on the human body?
Based on information from the top three websites on Google, the effects of titanium particles on the human body vary depending on factors such as particle size, concentration, and exposure duration. Titanium is generally considered biocompatible, making it a popular choice for medical implants. However, titanium particles released due to wear and corrosion can have biologically significant impacts.
- Particle Size and Concentration: The smaller the particles and the higher their concentration, the more likely they are to interact with biological tissues. Studies indicate that nano-sized titanium particles (<100 nm) are more readily taken up by cells compared to larger particles, potentially leading to cytotoxicity and inflammatory responses.
- Immune Response: Titanium particles can trigger an immune response, characterized by the activation of macrophages and the release of pro-inflammatory cytokines. This chronic inflammation can lead to a condition known as periprosthetic osteolysis, where bone tissue around the implant is resorbed, potentially leading to implant loosening and failure.
- Toxicity: In vitro studies have shown that at high concentrations, titanium particles can induce oxidative stress and cytotoxicity in various cell types, including osteoblasts and fibroblasts. The release of reactive oxygen species (ROS) from these particles can damage cellular components, leading to impaired cell function and viability.
- Systemic Distribution: While most titanium particles are likely to remain localized around the implantation site, there is evidence suggesting that they can enter systemic circulation, potentially accumulating in organs such as the liver, spleen, and lungs. Long-term effects of such systemic distribution are still under investigation, but current findings highlight the need for ongoing monitoring and research.
By integrating these findings with clinical monitoring protocols, healthcare providers can better predict and manage the effects of titanium particles on patient health.
Understanding the presence of titanium particles
To address the presence of titanium particles effectively, it is crucial to implement comprehensive clinical strategies. Regular monitoring through advanced imaging techniques and biomarker analysis can help detect early signs of periprosthetic osteolysis and inflammation. Additionally, developing and utilizing implant materials with enhanced biocompatibility or surface treatments to reduce particle generation can mitigate cytotoxicity and immune responses. Further research into the systemic distribution of titanium particles will enhance our understanding, guiding the development of targeted therapeutic interventions to minimize long-term health risks. Ensuring rigorous clinical follow-ups and patient education is essential in managing the effects of titanium particles on overall patient well-being effectively.
Impact on the implant site
The implantation of titanium-based prosthetics can result in various biological responses at the implant site, primarily driven by the interaction between titanium particles and the surrounding tissues. According to the top sources, including the National Center for Biotechnology Information (NCBI), PubMed, and reputable orthopedic journals, the primary impacts are:
- Local Inflammation and Osteolysis:
- Titanium particles can stimulate an inflammatory response, leading to periprosthetic osteolysis. This is often mediated by the recruitment of macrophages and subsequent release of pro-inflammatory cytokines such as TNF-α and IL-1β.
- In technical parameters, early detection of inflammation can be achieved through biomarkers like CRP (C-Reactive Protein) levels and advanced imaging techniques such as MRI and CT scans which monitor bone density and structure.
- Material Degradation and Wear:
- Long-term wear of titanium implants due to mechanical stress can lead to the generation of wear particles. This wear is quantified using parameters such as wear rate (measured in mm³/million cycles) and roughness average (Ra), which affects the rate of particle release.
- Surface treatments like anodization or the application of hydroxyapatite coatings can mitigate wear by improving the hardness and reducing the coefficient of friction.
- Adverse Tissue Reactions:
- The toxicity and immune response to titanium particles can manifest as fibrous encapsulation around the implant, leading to loosening and implant failure. Assessment of tissue response includes histological analysis and immunohistochemistry to identify the extent of fibrosis and cell infiltration.
In conclusion, addressing the impact on the implant site involves a combination of regular clinical monitoring, advanced imaging, and the use of enhanced implant materials. By understanding these parameters and their implications, healthcare providers can strategize effectively to mitigate adverse effects and enhance patient outcomes.
Long-term health effects
Long-term health effects of titanium implants are influenced by several factors, including material degradation, wear particles, and the body’s immune response. Chronic exposure to titanium wear particles can lead to systemic inflammation, potentially exacerbating conditions like osteolysis, which impacts bone density and implant stability. Studies highlight that prolonged exposure can also lead to the release of metal ions into the bloodstream, with potential nephrotoxic and hepatotoxic effects, though such occurrences are rare. Regular monitoring through biomarkers (e.g., CRP levels) and advanced imaging is crucial for early detection and management of adverse effects to improve patient outcomes.
Frequently Asked Questions (FAQs)
Q: What are the common side effects of titanium screws in the body?
A: Common side effects of titanium screws can include localized pain, inflammation, and allergic reactions. In some cases, patients with titanium implants may experience systemic effects on the human body, although these instances are rare because titanium is biocompatible.
Q: Is the titanium implant procedure safe?
A: Yes, the titanium implant procedure is generally considered safe. Titanium and titanium alloys are commonly used because of their inert properties and biocompatibility. However, it is essential to discuss potential risks and individual conditions with your surgeon.
Q: Can titanium implants corrode inside the body?
A: Titanium implants have high resistance to corrosion. The corrosion of titanium is minimal due to the formation of a stable oxide layer that protects the metal, making it a suitable choice for orthopedic implants and other medical devices.
Q: What are the effects of systemic titanium on the human body?
A: Systemic titanium effects are exceedingly rare because titanium, especially pure titanium, is inert and does not interact significantly with body tissues. Most patients do not experience any systemic effects from their implants.
Q: How can the problems caused by titanium implants be minimized?
A: Problems caused by titanium implants can be minimized through proper surgical technique during the titanium implant procedure, careful patient selection, regular follow-up, and prompt attention to any signs of complications such as pain, swelling, or infection.
Q: Are there any long-term effects of having titanium screws, rods, or implants?
A: Long-term effects are usually minimal due to the biocompatibility of titanium. However, some patients might experience minor issues like wear and tear over many years. Regular check-ups can help to monitor and address any long-term effects.
Q: What is the difference between titanium and titanium alloys in medical implants?
A: Titanium is a metal known for its strength and light weight. Titanium alloys combine titanium with other metals to enhance specific properties such as durability and strength. Both types are used in medical implants like bone screws and hip replacements due to their biocompatibility.
Q: Can titanium implants cause allergic reactions?
A: While titanium is inert and generally does not cause allergic reactions, some patients might still experience sensitivity. It’s crucial to discuss any known allergies or sensitivities with your doctor before undergoing a titanium implant procedure.
Q: What materials can titanium implants get combined with to enhance their properties?
A: Titanium implants can be combined with materials such as titanium dioxide and other elements to form titanium alloys. These combinations help in improving the overall strength, wear resistance, and durability of the implants.
Q: Are there any alternative materials to titanium for implants?
A: Yes, there are alternative materials such as stainless steel, cobalt-chromium alloys, and ceramic composites. However, titanium and titanium alloys are often preferred due to their superior biocompatibility and lower risk of adverse effects.