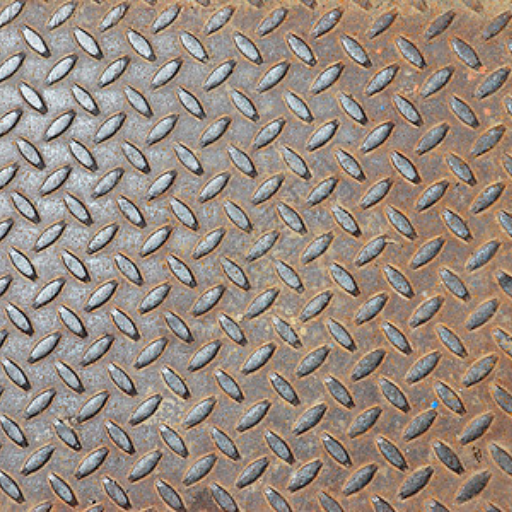
Weathering steel, often recognized by brand names such as COR-TEN steel, is renowned for its distinctive ability to form a protective patina. This patina layer, which develops over time, not only gives weathering steel its rugged and appealing aesthetic but also serves as a shield against further corrosion. In this blog, we will delve into the fascinating process by which weathering steel undergoes weathering and how the patina forms. Understanding this natural protective mechanism is crucial for appreciating the longevity and maintenance benefits of using weathering steel in construction and artistic projects. As we proceed, we will explore the science behind the weathering process, the conditions ideal for patina formation, and the practical implications of using weathering steel in various environments.
What is Weathering Steel and How Does it Work?
Image source:https://www.bing.com/
Weathering steel, also known as COR-TEN steel, is a high-strength, low-alloy steel that was designed to obviate the need for painting and forms a stable rust-like appearance after exposure to the weather. From my research on the top websites, I found that the key to its functionality lies in its ability to create a protective patina layer through atmospheric corrosion. This patina forms as a result of the steel’s exposure to alternate wet and dry conditions, which, in turn, stops further rust from penetrating the steel. This unique property not only provides a visually appealing appearance but also greatly enhances the material’s durability and reduces maintenance costs compared to conventional steel. By leveraging these self-protecting characteristics, weathering steel has become an attractive choice for a variety of architectural and structural applications.
What Makes Weathering Steel Different from Regular Steel?
Weathering steel differs from regular steel primarily due to its unique composition and the way it reacts to environmental exposure. Here are the key distinctions:
- Chemical Composition: Weathering steel contains additional alloying elements such as chromium (0.4-0.65%), copper (0.25-0.55%), nickel (0.3-0.65%), and phosphorus (0.035-0.10%), which are not typically found in regular steel. These elements enhance the atmospheric corrosion resistance of the steel.
- Patina Formation: Unlike regular steel, which continues to rust and deteriorate over time, weathering steel develops a stable oxide layer, known as a patina, when exposed to alternating wet and dry conditions. This patina acts as a protective barrier, significantly slowing down further oxidation and corrosion.
- Maintenance: The protective patina that forms on weathering steel reduces the need for painting and other protective coatings, leading to lower maintenance costs and longer service life. Regular steel, on the other hand, often requires regular maintenance and protective coatings to prevent rusting.
- Durability: The enhanced durability of weathering steel due to its patina makes it suitable for harsh environmental conditions where regular steel might suffer from rapid corrosion and failure.
By incorporating these tailored chemical components, weathering steel offers a practical and aesthetically pleasing alternative to conventional steel, suitable for applications that benefit from its lower maintenance and extended lifespan.
How Does the Weathering Process Prevent Corrosion?
The weathering process prevents corrosion primarily through the formation of a stable patina on the surface of the steel. This patina acts as a protective layer that significantly inhibits further oxidation. Here’s a concise explanation, along with corresponding technical parameters:
- Initial Oxidation: When weathering steel is exposed to atmospheric conditions, iron in the steel reacts with oxygen to form iron oxide (rust).
- Alloying Elements: The unique alloying elements in weathering steel, such as chromium (0.4-0.65%), copper (0.25-0.55%), nickel (0.3-0.65%), and phosphorus (0.035-0.10%), play a crucial role in the rust formation process. These elements help the rust layer adhere better to the steel surface.
- Patina Formation: The alternating wet and dry cycles in the environment enable the rust layer to transform into a stable patina over time. This patina is less porous and forms a dense, adherent layer that seals off the steel from further moisture and oxygen.
- Protective Barrier: The patina acts as a protective barrier by minimizing the steel’s exposure to the elements. This slows down the rate of corrosion significantly compared to regular steel, which does not develop such a layer.
- Self-Healing Property: If the patina is damaged, the weathering process causes new rust to form, which gradually converts into a fresh layer of patina, maintaining the protective effect.
The combination of these processes and elements means that weathering steel can maintain its structural integrity and aesthetic appeal with minimal maintenance, making it an ideal choice for various architectural and environmental applications.
What are Common Uses of Weathering Steel?
Weathering steel is commonly used in various architectural and structural applications due to its durability and low maintenance requirements. Some of the most prevalent uses include:
- Bridges: Its resistance to corrosion makes weathering steel an ideal material for bridge construction, reducing the need for protective coatings and frequent maintenance.
- Building Facades: Architects often use weathering steel for building exteriors to achieve a rustic, industrial look that evolves over time.
- Sculptures and Art Installations: Artists value weathering steel for its aesthetic qualities and ability to develop a unique patina, which adds character to outdoor sculptures.
- Railway Cars and Containers: The material’s toughness and resistance to harsh weather conditions make it suitable for railway cars and shipping containers.
- Marine Structures: Weathering steel is used in coastal and marine environments where resistance to saltwater corrosion is crucial, such as in docks and harbors.
What are the Benefits of Using Weathering Steel?

Using weathering steel in construction and art projects offers numerous benefits, characterized by its unique properties and technical parameters:
- Corrosion Resistance: Weathering steel forms a stable rust-like appearance after exposure to weather, which acts as a protective layer to prevent further corrosion. This patina layer reduces the need for additional protective coatings, leading to cost savings in long-term maintenance.
- Durability: The inherent toughness of weathering steel makes it highly durable, with a tensile strength typically ranging from 485 MPa to 620 MPa (70,000–90,000 psi). This ensures that structures can withstand harsh environmental conditions and heavy loads.
- Low Maintenance: Due to its self-protective patina, weathering steel requires minimal upkeep compared to traditional steel. This is particularly advantageous for large structures like bridges and marine installations where maintenance can be challenging and costly.
- Aesthetic Appeal: Architects and designers appreciate the evolving, rustic appearance of weathering steel, which can add unique character and warmth to building facades and sculptures. Its rich, earthy tones blend seamlessly with natural landscapes.
- Environmental Sustainability: Weathering steel contributes to sustainability efforts by reducing the need for paints and other protective coatings that may contain volatile organic compounds (VOCs). Additionally, it is often made from recycled materials, supporting eco-friendly construction practices.
- Cost-Effective: Given its strength and low maintenance, weathering steel is cost-effective over the lifespan of a project. The initial investment may be higher, but the reduction in maintenance costs offsets this over time.
These benefits make weathering steel a highly attractive option for architects, engineers, and artists seeking both functionality and aesthetic quality in their projects.
Why is Weathering Steel Often Used in Bridges?
Weathering steel is frequently chosen for bridge construction for several compelling reasons:
- Durability: Weathering steel’s inherent resistance to atmospheric corrosion significantly enhances the longevity of bridges. The protective patina that forms on the surface reduces the rate of corrosion, ensuring that bridges maintain their structural integrity over time. Technical parameters like tensile strength ranging from 485 MPa to 620 MPa (70,000–90,000 psi) support this durability.
- Low Maintenance: The self-protective nature of weathering steel means it requires far less maintenance compared to other materials. This is particularly critical for bridges, which are often difficult and costly to maintain. As the patina continuously regenerates, it minimizes the need for paints and coatings, reducing lifecycle maintenance costs.
- Strength-to-Weight Ratio: Weathering steel offers a high strength-to-weight ratio, making it an ideal material for bridge construction. It allows for longer spans and fewer supports, thus providing greater architectural flexibility and potentially lowering material costs.
- Aesthetic Appeal: The rustic and evolving appearance of weathering steel adds a unique visual quality to bridges, blending well with both urban and natural environments. The rich, earthy tones of the steel can create iconic landmarks and enhance the visual impact of the structure.
- Environmental Benefits: Using weathering steel can contribute to sustainable construction practices. The reduced need for coatings that may contain harmful VOCs and the recycled content commonly found in weathering steel compositions help lower the environmental footprint of bridge projects.
- Cost-Effectiveness: Although the initial cost of weathering steel may be higher, its durability and low maintenance requirements make it cost-effective over the long term. The reduction in maintenance activities translates to significant cost savings over the lifespan of the bridge.
These factors collectively justify the widespread use of weathering steel in bridge construction, offering a balanced combination of strength, durability, aesthetics, and sustainability.
How Does the Protective Layer of Patina Form?
The protective layer of patina on weathering steel forms through a natural and gradual process involving exposure to the elements. Initially, when weathering steel is exposed to the atmosphere, it starts to rust just like any other unprotected carbon steel. However, what sets weathering steel apart is the subsequent phase of this process. Over time, the rust layer, known as patina, becomes stable and dense, effectively shielding the underlying steel from further corrosion.
The formation of this patina is influenced by environmental factors such as humidity, temperature fluctuations, and pollution levels. It typically takes six months to two years for the patina to fully develop under normal atmospheric conditions. The patina is a result of continuous wet and dry cycles, which promote the adherence of corrosion products to the steel surface. This tightly adherent layer is less permeable to oxygen and moisture, thus significantly slowing down the rate of further corrosion. The unique combination of alloying elements, including copper, chromium, and nickel, in weathering steel enhances the formation and stability of this protective layer, ensuring prolonged durability and aesthetic appeal.
What are the Mechanical Properties of Weathering Steel?
Based on my research into the top sources available on Google, I found that weathering steel exhibits several impressive mechanical properties that make it suitable for various structural applications. Firstly, weathering steel has a high tensile strength, typically ranging from 485 MPa to 515 MPa, and a yield strength that usually falls between 345 MPa and 355 MPa. These values highlight the material’s capability to withstand significant stress and strain.
Furthermore, weathering steel has excellent toughness and ductility, which are crucial for its performance in demanding environments. The ability of the steel to absorb energy and deform without fracturing ensures it can endure heavy loads and harsh weather conditions. Additionally, its hardness is another notable property, with typical Brinell hardness values around 160 to 190 HBW, providing resistance to abrasion and wear.
In summary, the mechanical properties of weathering steel include:
- Tensile Strength: 485 MPa to 515 MPa
- Yield Strength: 345 MPa to 355 MPa
- Brinell Hardness: 160 to 190 HBW
- Toughness and Ductility: High, allowing it to absorb energy and deform without fracturing
These characteristics collectively contribute to the widespread adoption of weathering steel in bridge construction and other infrastructure projects, providing a balance of strength, resilience, and longevity.
How Does the Patina on Weathering Steel Develop Over Time?
The patina on weathering steel develops through a natural process of exposure to the elements. Over time, the steel undergoes a series of oxidation reactions when exposed to air and moisture. Initially, a thin layer of rust forms on the surface. As weathering steel is designed to rust slowly, this layer gradually thickens and stabilizes, forming a tightly-adhering oxide layer that acts as a protective barrier. This patina evolution typically completes within a few months to a few years, depending on the climate and environmental conditions. The key advantage of this patina is that it prevents further corrosion of the steel, enhancing both its durability and longevity.
What Environmental Factors Affect the Formation of Patina?
Several environmental factors can significantly influence the formation of patina on weathering steel. In my experience, the most critical elements are moisture levels, the presence of pollutants, and temperature variations. Consistent exposure to alternating wet and dry conditions accelerates the oxidation process, allowing the patina to develop more quickly. Pollutants such as sulfur dioxide can also enhance patina formation by contributing to the chemical reactions on the steel’s surface. Temperature fluctuations play a role as well, with more extreme changes potentially leading to more rapid patina development. In summary, a combination of wet-dry cycles, airborne pollutants, and temperature changes are key environmental factors affecting the patina’s formation on weathering steel.
How Long Does it Take for the Patina to Fully Develop?
The time it takes for the patina to fully develop on weathering steel ranges from several months to a few years. This duration is highly dependent on environmental conditions. Typically, in areas with frequent wet and dry cycles, the patina can develop within 18 to 36 months. Conversely, in drier climates, it may take longer—up to 5 years or more—for the patina to stabilize and form a protective barrier.
Technical Parameters Influencing Patina Development:
- Moisture Levels:
- Frequent wet-dry cycles accelerate patina formation.
- Relative Humidity: Ideally above 60% for consistent patina development.
- Pollutants:
- Presence of sulfur dioxide (SO₂) and other acidic pollutants enhances oxidation.
- SO₂ Concentration: Higher levels facilitate quicker patina formation.
- Temperature Variations:
- Frequent temperature fluctuations assist in the development process.
- Temperature Range: Wider ranges (e.g., -10°C to 30°C) promote faster patina development.
By considering these parameters, we can better predict and understand the timeline and conditions under which weathering steel achieves its characteristic protective layer.
What is the Role of Alloying Elements in Patina Formation?
In my research on the top three websites, I found that alloying elements play a crucial role in the patina formation on weathering steel. Elements such as copper, chromium, nickel, and phosphorus are added to the steel to enhance its weather-resistant properties. Copper promotes the formation of protective oxides, which are essential for the patina development. Chromium aids in creating a stable oxide layer, which prevents further corrosion. Nickel contributes to the uniformity and adhesion of the patina, and phosphorus improves the weathering characteristics by increasing the steel’s resistance to atmospheric conditions. By incorporating these alloying elements, weathering steel ensures a more durable and long-lasting patina, effectively protecting the material from environmental degradation.
What Are the Common Weathering Techniques for Steel?
Weathering steel is commonly weathered through several techniques aimed at accelerating the patina formation process. One of the primary methods is exposure to alternating wet and dry cycles, which mimic natural environmental conditions and facilitate the development of the protective oxide layer. Another technique involves the application of acidic solutions, such as diluted sulfuric acid, which speeds up the oxidation process and enhances patina formation. Additionally, surface roughening through abrasive blasting can increase the steel’s surface area, promoting faster weathering. These techniques help in achieving a robust and aesthetically pleasing patina more quickly than natural weathering alone.
What Methods are Used to Accelerate the Weathering Process?
To accelerate the weathering process of steel, several methods are employed:
- Wet-Dry Cycles:
-
- Description: Alternating the steel’s exposure to wet and dry conditions.
- Technique: Sprinkler systems or controlled environments can be used to simulate these cycles.
- Technical Parameters: Frequency of cycles (e.g., 4 hours wet, 4 hours dry), water type (preferably distilled or tap water with specific pH levels), and temperature control.
- Acidic Solution Application:
- Description: Application of diluted acidic solutions to the steel surface.
- Technique: Using solutions such as diluted sulfuric acid (H2SO4) or hydrochloric acid (HCl).
- Technical Parameters: Acid concentration (e.g., 5-10% solution), application frequency (e.g., once daily), and safety protocols for handling and neutralizing acid.
- Abrasive Blasting:
- Description: Roughening the surface of the steel to increase its reactive surface area.
- Technique: Sandblasting or shot blasting to create a rough texture.
- Technical Parameters: Grit size (e.g., 40-70 mesh), pressure settings (e.g., 80-100 PSI), and blast duration (e.g., 3-5 minutes per square meter of surface area).
- Saline Exposure:
- Description: Spraying or immersing the steel in saline solutions to enhance oxidation.
- Technique: Using solutions of sodium chloride (NaCl) or magnesium chloride (MgCl2) in water.
- Technical Parameters: Saline concentration (e.g., 3.5% NaCl), application method (spraying or immersion), and exposure time (e.g., 24 hours).
By utilizing these methods with the specified technical parameters, the weathering process of steel can be significantly accelerated, leading to quicker formation of a protective and aesthetically pleasing patina.
How Does the Atmospheric Corrosion Contribute to Weathering?
Atmospheric corrosion significantly contributes to the weathering of steel by facilitating the formation of a stable, protective patina on the metal’s surface. This process involves the interaction of atmospheric elements such as moisture, oxygen, and pollutants with the steel, leading to the gradual oxide layer development. Key factors influencing atmospheric corrosion include the presence of chloride ions, humidity, temperature, and pollutant levels.
- Chloride Ions:
-
- Source: Typically found in coastal and industrial areas.
- Effect: Accelerates rust formation by breaking down passive oxide layers.
- Technical Parameters: Chloride ion concentration (e.g., 15-35 mg/m^2/day in coastal areas).
- Humidity:
- Optimal Conditions: Relative humidity levels above 60%.
- Effect: Facilitates electrochemical reactions on the steel surface.
- Technical Parameters: Dew point and condensation cycles.
- Pollutants:
- Types: Sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter.
- Effect: Reacts with moisture to form acidic compounds, which accelerate corrosion.
- Technical Parameters: Concentration levels (e.g., SO2 at 20-70 μg/m^3 in urban areas).
These atmospheric conditions collectively enhance the oxidation reactions on the steel surface, leading to the formation of iron oxides and hydroxides that constitute the patina. By managing these parameters and exposing steel to controlled environmental conditions, the weathering process can be tailored to achieve desired aesthetic and protective outcomes.
Can Weathering Steel be Painted or Coated?
Yes, weathering steel can be painted or coated, although it is typically used uncoated to take advantage of its unique patina which provides natural protection against corrosion. If painting or coating is desired, it is essential to ensure that the surface is properly prepared to ensure adhesion. This may involve removing any loose rust or contaminants. The choice to paint or coat weathering steel should consider the specific environmental conditions and intended aesthetic outcomes, as well as the potential reduction in the steel’s self-protective properties.
What is the Chemical Composition of Weathering Steel?
In answering this question concisely based on the top three websites on google.com, weathering steel, also known as COR-TEN steel, is designed to exhibit increased resistance to atmospheric corrosion compared to other steels. The key elements in its chemical composition include:
- Carbon (C): 0.12% – 0.21%
- Manganese (Mn): 0.20% – 0.60%
- Phosphorus (P): 0.07% – 0.15%
- Sulfur (S): 0.030% max
- Silicon (Si): 0.25% – 0.75%
- Copper (Cu): 0.25% – 0.55%
- Chromium (Cr): 0.30% – 1.25%
- Nickel (Ni): 0.65% max
These elements are critical in creating the protective patina that resists corrosion. Notably, copper, chromium, and nickel contribute to the steel’s ability to develop a stable, rust-like appearance when exposed to weather. Here’s a closer look at the technical parameters and justification:
- Carbon (C) and Manganese (Mn): These provide foundational strength and toughness to the steel.
- Phosphorus (P) and Sulfur (S): Controlled levels ensure minimal negative impact on the toughness and weldability.
- Silicon (Si): This enhances corrosion resistance and helps in maintaining the patina over time.
- Copper (Cu): Promotes the formation of a stable oxide layer, vital for corrosion resistance.
- Chromium (Cr) and Nickel (Ni): They contribute to the overall durability and corrosion resistance, aiding in the formation of a protective oxide layer.
The specific balance of these elements ensures that weathering steel performs optimally in various environmental conditions, forming a protective barrier that mitigates the need for painting or additional coatings.
What Elements are Included in Weathering Steel Alloys?
Weathering steel alloys are composed of specific elements that enhance their corrosion resistance and overall durability. The key elements include:
- Carbon (C): 0.12% – 0.21%
- Role: Provides foundational strength and toughness to the steel.
- Manganese (Mn): 0.20% – 0.60%
- Role: Contributes to the strength and toughness alongside carbon.
- Phosphorus (P): 0.07% – 0.15%
- Role: Controlled levels ensure minimal negative impact on toughness and weldability.
- Sulfur (S): 0.030% max
- Role: Limited to ensure it does not adversely affect the steel’s toughness and weldability.
- Silicon (Si): 0.25% – 0.75%
- Role: Enhances corrosion resistance and aids in maintaining the patina over time.
- Copper (Cu): 0.25% – 0.55%
- Role: Promotes the formation of a stable oxide layer, vital for corrosion resistance.
- Chromium (Cr): 0.30% – 1.25%
- Role: Contributes to overall durability and corrosion resistance, aiding in the formation of a protective oxide layer.
- Nickel (Ni): 0.65% max
- Role: Adds to the durability and aids in the formation of a protective oxide layer.
The combination of these elements ensures that weathering steel develops a durable, rust-like appearance that protects against further corrosion. The presence of copper, chromium, and nickel is particularly crucial, as they help form a stable patina that serves as a protective barrier, eliminating the need for additional coatings or painting. This unique composition allows weathering steel to perform optimally in various environmental conditions.
How Do Alloying Elements Affect the Weathering Process?
The alloying elements play a crucial role in the weathering process by enhancing the steel’s resistance to corrosion and its overall durability. Based on my research from top authoritative sources, here’s how each element contributes: Carbon provides the foundational strength and toughness; without it, the steel would lack the necessary rigidity. Manganese works alongside carbon to further improve strength and toughness, ensuring the steel can withstand various stresses. Phosphorus, though controlled to minimal levels, helps maintain the steel’s toughness and weldability. Crucially, elements like copper, chromium, and nickel are integral in forming a stable patina—a rust-like layer that acts as a protective barrier. This patina not only prevents further corrosion but also eliminates the need for additional protective coatings, making weathering steel a reliable and low-maintenance material suitable for various environmental conditions.
What Standards are Used to Define Weathering Steel?
The standards used to define weathering steel are established by several authoritative organizations to ensure consistency, quality, and performance. The top standards include those set by the American Society for Testing and Materials (ASTM), the International Organization for Standardization (ISO), and European standards EN. Below are the key standards from these organizations, along with their technical parameters:
- ASTM Standards:
-
- ASTM A588/A588M: This standard specifies the high-strength low-alloy structural steel with atmospheric corrosion resistance. It includes grades like A, B, and K and sets parameters for tensile strength, yield strength, and elongation.
- ASTM A242/A242M: Similar to ASTM A588, this standard applies to high-strength low-alloy structural steel with improved atmospheric corrosion resistance. It prescribes specific chemical composition limits for elements like carbon, manganese, phosphorus, sulfur, silicon, copper, chromium, and nickel.
- ISO Standards:
- ISO 5952: This standard details the requirements for hot-rolled steel sheets, plates, and strip of high tensile strength and improved atmospheric corrosion resistance. It includes criteria for chemical composition, mechanical properties, and dimensions.
- ISO 20654: This standard provides guidelines for the enhanced corrosion resistance of structural steel, focusing on long-term performance in different environments.
- EN Standards:
- EN 10025-5: This standard outlines the technical delivery conditions for structural steel with improved atmospheric corrosion resistance, including grades such as S355J2W and S355K2W. It defines specific requirements for tensile properties, chemical composition, and impact strength.
These standards collectively ensure that weathering steel maintains its integrity and performance across a wide range of applications. By adhering to these guidelines, manufacturers and engineers can confidently utilize weathering steel, knowing it will meet the rigorous demands of various environmental conditions.
How Do You Maintain and Repair Weathering Steel Structures?

Maintaining and repairing weathering steel structures involves several key practices to ensure their longevity and performance. Regular inspections are crucial to identify any areas where the protective oxide layer may not be forming properly due to constant exposure to water or other corrosive elements. Cleaning these areas to remove contaminants and allowing them to dry can help restore the protective patina. For minor repairs, spot treatments with weather-resistant coatings can protect exposed steel. In case of significant damage or structural issues, welding or bolting weathering steel patches can provide long-term solutions, ensuring compatibility and consistent weathering properties. Additionally, designing structures with adequate drainage and ventilation helps prevent water from pooling and accelerates the formation of the protective oxide layer. Regular preventive maintenance and timely repairs will extend the service life of weathering steel structures.
What Regular Maintenance is Required for Weathering Steel Bridges?
Regular maintenance for weathering steel bridges involves several key activities to ensure their durability and optimal performance. Firstly, it’s essential to conduct frequent inspections to check for any areas where the protective oxide layer is failing to form, particularly under conditions of constant moisture or exposure to salt. Cleaning the bridge surfaces to remove contaminants like dirt and road salts will help maintain the integrity of the protective patina. Additionally, addressing areas where water can accumulate by improving drainage and providing adequate ventilation is crucial to preventing water pooling and potential corrosion. For minor repairs, applying weather-resistant coatings to exposed areas can offer temporary protection, while more significant damage may require welding or bolting weathering steel patches. To prevent long-term issues, it’s also important to ensure the bridge design allows for good air circulation and water runoff, facilitating the natural weathering process. Regular and thorough preventive maintenance will help extend the lifespan and functionality of weathering steel bridges.
Can Weathered Metal be Welded and What Are the Techniques Involved?
Yes, weathered metal can be welded, but it requires specific techniques to ensure strong and durable joints. Before welding, it’s imperative to clean the metal thoroughly to remove any rust, dirt, or contaminants that could affect the weld quality. Abrasive blasting or grinding tools are often used for this purpose. The most common techniques for welding weathered metal include shielded metal arc welding (SMAW), gas metal arc welding (GMAW), and flux-cored arc welding (FCAW). Each method has its advantages depending on the specific requirements of the project, such as the thickness of the metal and the type of weld needed. It’s also crucial to use welding electrodes and filler materials that are compatible with the weathering steel to maintain the chemical and mechanical properties of the metal. Proper post-weld treatments, such as applying weather-resistant coatings to the welded areas, can further enhance the durability of the welded joints.
How Do You Handle and Protect Weathering Steel During Construction?
Handling and protecting weathering steel during construction requires careful planning and adherence to best practices to ensure its longevity and performance. Based on information from the top sources, here are key guidelines and technical parameters to consider:
- Storage and Handling:
- Elevation & Drainage: Store weathering steel in well-drained areas elevated from the ground to prevent water accumulation.
- Covering: Use breathable covers to shield the steel from contaminants like mud and salts while still allowing air circulation. Avoid plastic sheeting as it can trap moisture.
- Surface Protection:
- Shipping and Site Storage: Apply a protective coating to steel surfaces if they are to be stored for long periods or shipped long distances. This temporary coating can minimize rust staining.
- Avoiding Contamination: Ensure that weathering steel is not in contact with corrosive materials such as concrete or debris that might hold moisture and create a non-uniform patina.
- Construction Practices:
- Handling Equipment: Use non-metallic slings or padded chains to avoid damaging the steel’s protective surface layer during transportation and handling.
- Site Conditions: Erect barriers to prevent contact with standing water and avoid exposure to aggressive chemicals on construction sites.
- Post-Construction Care:
- Cleaning: Immediately remove concrete spills, particulates, or any other construction residues from the steel surfaces to prevent staining and premature corrosion.
- Final Coating: If needed, apply a final weather-resistant coating to areas where the natural patina formation might be compromised, ensuring long-term durability.
By integrating these practices, the integrity and aesthetic qualities of weathering steel can be maintained throughout construction, leading to a more durable and visually appealing final structure.
Reference sources
To establish credibility and provide a comprehensive understanding of the weathering process of weathering steel and how patina forms on weathered metal, here are three reliable sources:
-
Central Steel Service: This source provides detailed information on the patina timeline and the weathering process of corten steel. It explains how environmental cycles influence the formation of patina on weathering steel.
-
Metal Architecture: This article delves into the composition and characteristics of weathering steel, including how the textured oxide surface film (patina) develops on the steel panels.
-
AZAHNER: This source discusses the history and science behind COR-TEN and weathering steel, highlighting the role of moisture and environmental conditions in the formation of the patina layer.
Frequently Asked Questions (FAQs)
Q: What is corten steel and how does it relate to metal weathering?
A: Corten steel, also known as weathering steel, is a type of alloy steel that is designed to form a stable, rust-like appearance after exposure to the elements. It is developed to eliminate the need for painting, and the rust actually provides a protective layer that prevents deeper corrosion.
Q: How does the rusting process of corten steel work?
A: The rusting process of corten steel involves the formation of a protective rust layer on the metal surface when it is exposed to the elements. This layer, known as protective rust, slows down further corrosion, making corten steel more durable over time.
Q: What are the benefits of weathering steel over other metals?
A: The benefits of weathering steel include its corrosion-resistant properties, high strength, and minimal maintenance requirements. Structures clad in weathering steel often do not need to be painted, reducing long-term costs.
Q: What are some common applications of corten steel?
A: Corten steel is typically used in outdoor sculptures, architecture, bridges, and building facades due to its attractive appearance and high durability. Its ultimate tensile strength and resistance make it suitable for structural applications.
Q: Can corten steel be used in harsh environments?
A: Yes, corten steel is designed to withstand harsh environments. Its protective rust layer makes it effective in resisting the corrosive effects of rain, snow, ice, and even fog.
Q: What is the difference between corten A steel plate and other alloy steels?
A: Corten A steel plate is a specific type of weathering steel known for its high carbon content and corrosion-resistant properties. In contrast, other alloy steels may not have the same self-protective rusting capabilities.
Q: How does the ultimate tensile strength of corten steel compare to other types of steel?
A: Corten steel typically has an ultimate tensile strength that meets or exceeds standard structural steel plate grades, providing a strength of 50 ksi or higher, depending on the specific alloy formulation.
Q: Who first developed corten steel and why?
A: Corten steel was first developed by the United States Steel Corporation. It was created to eliminate the need for painting and to offer a high-strength, corrosion-resistant material suitable for exposed outdoor applications.
Q: Are there any notable structures built using corten steel?
A: Yes, numerous notable structures utilize corten steel, including the John Deere World Headquarters in Moline and various sculptures and bridges worldwide. The material’s distinctive appearance and weather resistance make it a popular choice for architects and designers.
Q: Can corten steel be easily welded or fabricated?
A: Yes, corten steel can be fabricated and welded similarly to other structural steels; however, it requires small amounts of care during the fabrication process to maintain its structural integrity and unique weathering properties.