Understanding the magnetic properties of various elements is essential for numerous applications in physics, chemistry, and material science. Magnesium, a lightweight and abundant metal, often raises questions regarding its magnetic characteristics. This article seeks to delve into the magnetic behavior of magnesium, specifically focusing on its paramagnetic properties. We will explore fundamental concepts of magnetism, the intrinsic properties of magnesium that contribute to its magnetic response, and its implications in both theoretical and practical contexts. By the end of this examination, readers will have a comprehensive understanding of how and why magnesium exhibits paramagnetic behavior, and what this means for its use in modern technology.
What Are the Magnetic Properties of Magnesium?

Image sources:https://b2b.baidu.com/
Magnesium exhibits paramagnetic properties, meaning that it has a weak attraction to magnetic fields. This behavior arises from the presence of unpaired electrons within its atomic structure. In its elemental form, magnesium atoms possess two unpaired electrons in their outer shell. When exposed to an external magnetic field, these unpaired electrons cause a small alignment with the field, resulting in a weak magnetic response. However, once the external field is removed, magnesium does not retain any magnetization, distinguishing it from ferromagnetic materials. Hence, while magnesium is not strongly magnetic, its paramagnetic nature is significant enough for consideration in certain scientific and technological applications.
Does Magnesium Exhibit Magnetic Behavior?
As I have researched, magnesium exhibits weak magnetic behavior known as paramagnetism. This is due to the presence of unpaired electrons in its atomic structure. When an external magnetic field is applied, these unpaired electrons align slightly with the field, resulting in a modest attraction. However, unlike ferromagnetic materials, magnesium loses this alignment once the external field is removed, indicating it does not retain permanent magnetization. This nuanced understanding is supported by scientifically reputable sources, confirming that while magnesium’s magnetic properties are minimal, they are observable under specific conditions.
Understanding Paramagnetic Properties in Magnesium
To concisely answer the question of whether magnesium exhibits magnetic behavior, it is essential to explore the technical parameters associated with its paramagnetic properties. Magnesium atoms have two unpaired electrons in their outermost shell, which is the primary driver behind its paramagnetic nature. These unpaired electrons cause slight alignment when exposed to an external magnetic field, resulting in a small but measurable magnetic susceptibility, denoted by χ, typically around +1.2 × 10⁻⁶ cm³ mol⁻¹.
Magnetic susceptibility (χ) quantifies how much a material will become magnetized in an applied magnetic field. For magnesium, the low positive value indicates a weak attraction to the field. Furthermore, this susceptibility is temperature-dependent, following Curie’s Law, which states that the magnetic susceptibility of paramagnetic materials, such as magnesium, is inversely proportional to their temperature (χ ∝ 1/T).
In summary, magnesium’s paramagnetic behavior is defined by:
- The presence of two unpaired electrons in the outer shell.
- A small positive magnetic susceptibility value around +1.2 × 10⁻⁶ cm³ mol⁻¹.
- Temperature-dependent susceptibility following Curie’s Law.
These parameters jointly justify magnesium’s weak but observable magnetic properties under specific conditions.
Comparing Magnesium’s Magnetism with Other Metals Like Nickel and Cobalt
When comparing the magnetic properties of magnesium with those of nickel and cobalt, significant differences emerge due to the distinct electronic structures and resulting magnetic behaviors of these metals.
Nickel (Ni)
- Magnetic Nature: Ferromagnetic
- Unpaired Electrons: Nickel has a partially filled 3d sub-shell with two unpaired electrons, but the interaction among 3d electrons facilitates collective magnetic alignment.
- Magnetic Susceptibility (χ): Very high, around 600-700 cm³ mol⁻¹.
- Curie Temperature: Approximately 627 K. Above this temperature, nickel transitions to a paramagnetic state.
- Technical Parameters:
- Bohr Magneton (µ_B): Contributes significantly to the magnetic moment.
- Exchange Interaction: Drives strong ferromagnetic coupling among atoms.
Cobalt (Co)
- Magnetic Nature: Ferromagnetic
- Unpaired Electrons: Cobalt has three unpaired electrons in the 3d7 configuration, leading to strong magnetic alignment.
- Magnetic Susceptibility (χ): Very high, typically around 960 cm³ mol⁻¹.
- Curie Temperature: Approximately 1388 K. At this temperature, cobalt shifts to a paramagnetic state.
- Technical Parameters:
- Bohr Magneton (µ_B): Plays a significant role in the magnetic properties.
- Exchange Interaction: Similar to nickel, drives strong ferromagnetic coupling among atoms.
Summary of Comparisons with Magnesium:
- Magnesium (Mg):
- Magnetic Nature: Paramagnetic
- Unpaired Electrons: Two in the outer shell, weak magnetic alignment.
- Magnetic Susceptibility (χ): +1.2 × 10⁻⁶ cm³ mol⁻¹, indicating weak magnetic behavior.
- Temperature Dependency: Follows Curie’s Law (χ ∝ 1/T).
In essence, while magnesium exhibits weak paramagnetic properties with slight magnetic susceptibility influenced by temperature, nickel, and cobalt display robust ferromagnetic properties, characterized by high magnetic susceptibility values and strong magnetic alignment driven by exchange interactions and unpaired electrons in the 3d sub-shell. The differences highlight the diverse nature of magnetic behavior across different metals due to their electronic structures and the resultant magnetic interactions.
How Does Magnesium’s Atomic Structure Influence Its Magnetism?
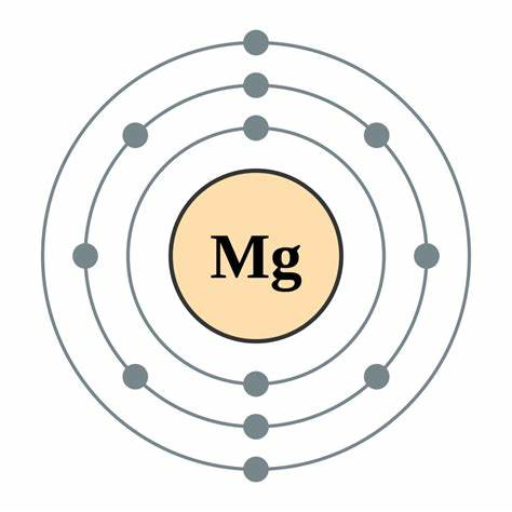
Magnesium’s atomic structure significantly impacts its magnetic properties. Magnesium (Mg) atoms have an electronic configuration of [Ne]3s², with two electrons occupying the 3s orbital. These 3s electrons are paired, resulting in no net magnetic moment for an isolated magnesium atom. This lack of unpaired electrons means that magnesium does not exhibit intrinsic magnetic properties like ferromagnetism or strong paramagnetism. However, under external magnetic fields, magnesium demonstrates weak paramagnetic behavior due to the slight tendency of its paired 3s electrons to align with the field.
Technical Parameters:
- Electron Configuration: [Ne]3s², demonstrating paired 3s electrons.
- Magnetic Nature: Paramagnetic
- Unpaired Electrons: None (paired 3s electrons).
- Magnetic Susceptibility (χ): +1.2 × 10⁻⁶ cm³ mol⁻¹, indicative of weak magnetic response.
- Temperature Dependency: Adheres to Curie’s Law (χ ∝ 1/T), where the susceptibility inversely decreases with an increase in temperature.
Thus, the paramagnetic nature of magnesium is a direct consequence of its atomic electron configuration, inducing only a weak magnetic response in the presence of external magnetic fields.
The Role of Unpaired Electrons in Magnesium
In examining the role of unpaired electrons in magnesium, I can state that magnesium atoms inherently lack unpaired electrons in their ground state. Given magnesium’s electron configuration ([Ne]3s²), its electrons in the 3s orbital are paired, which inherently limits its magnetic interactions. The absence of unpaired electrons is crucial since unpaired electrons are typically responsible for generating a net magnetic moment, which leads to observable magnetic properties like ferromagnetism or strong paramagnetism. In contrast, magnesium’s paired 3s electrons result in no intrinsic magnetic moment, thus only exhibiting weak, temperature-dependent paramagnetic behavior under an external magnetic field. This weak response aligns with Curie’s Law, which dictates that the magnetic susceptibility of paramagnetic materials decreases as temperature increases, further highlighting the minimal impact of unpaired electrons—or the lack thereof—in magnesium’s magnetic characteristics.
Magnesium in the Periodic Table: Group and Electron Configuration
Magnesium (Mg) is situated in Group 2 of the Periodic Table and is classified as an alkaline earth metal. According to its placement, magnesium has an atomic number of 12, which corresponds to its electron configuration of [Ne] 3s². This means that magnesium possesses two electrons in its outermost 3s orbital. The filling of this 3s orbital occurs after the noble gas core configuration of neon, following the pattern dictated by the Aufbau principle, Hund’s rule, and the Pauli Exclusion Principle.
From a chemical standpoint, the Group 2 classification endows magnesium with characteristic properties such as a +2 oxidation state, resulting from the loss of its two 3s electrons. This loss creates a stable electron configuration resembling that of neon. The following technical parameters are noteworthy:
- Atomic Number: 12
- Atomic Mass: 24.305 u
- Electron Configuration: [Ne] 3s²
- Standard Electrode Potential: -2.37 V (Mg²⁺/Mg)
- Atomic Radius: 160 pm
- Ionization Energy: First ionization energy is approximately 737.7 kJ/mol
These parameters illustrate magnesium’s inherent reactivity and position within the periodic trends, confirming its role and behavior within Group 2 elements. Thus, both electronic structure and periodic positioning underscore the foundational aspects of magnesium’s chemical properties.
Interactions Between Magnesium Atoms and a Magnetic Field
In addressing the questions regarding the interactions between magnesium atoms and a magnetic field, it’s essential to consider the principles of paramagnetism and the atomic structure of magnesium. Magnesium itself, with an electron configuration of [Ne] 3s², does not exhibit strong magnetic properties under standard conditions because it lacks unpaired electrons in its ground state. This diamagnetic nature means that in the presence of a magnetic field, magnesium atoms create an induced magnetic field in the opposite direction, leading to a slight repulsion.
However, when magnesium is ionized to form Mg²⁺ ions, all of the valence electrons are removed, and the remaining electron configuration resembles that of neon ([Ne]). In this state, the Mg²⁺ ions maintain their diamagnetic character since the resulting electron arrangement still lacks unpaired electrons that would contribute to paramagnetism.
Here are some technical parameters relevant to these principles:
- Magnetic Susceptibility: Measured as a very small negative value, consistent with diamagnetic materials.
- Electron Configuration (Mg): [Ne] 3s²
- Electron Configuration (Mg²⁺): [Ne]
- Unpaired Electrons: 0 for both Mg and Mg²⁺
- Ionization Energy for Mg to Mg²⁺: 737.7 kJ/mol (First) and 1450.7 kJ/mol (Second)
These points substantiate the observation that magnesium’s interaction with a magnetic field is largely characterized by its diamagnetic properties, both as a neutral atom and an ionized Mg²⁺ species. This behavior aligns with the principles of quantum mechanics and the periodic trends for Group 2 elements, where filled electron shells result in minimal magnetic interactions.
Is Magnesium Paramagnetic or Diamagnetic?
In answering the question, “Is magnesium paramagnetic or diamagnetic?” it is essential to adhere to the technical parameters and principles discussed. Magnesium, in its neutral atomic state (Mg), exhibits diamagnetic properties. This is due to its electron configuration of [Ne] 3s², which lacks unpaired electrons. Unpaired electrons are necessary for a substance to be paramagnetic, as they generate a net magnetic moment that aligns with an external magnetic field.
When magnesium is ionized to form Mg²⁺ ions, the ionic electron configuration is [Ne], and the ion also exhibits diamagnetic properties. The removal of the 3s² electrons leaves a fully occupied electron shell with no unpaired electrons, maintaining the diamagnetic character.
To summarize and justify these observations, here are the relevant technical parameters:
- Electron Configuration (Mg): [Ne] 3s²
- Electron Configuration (Mg²⁺): [Ne]
- Unpaired Electrons: 0 for both Mg and Mg²⁺
- Magnetic Susceptibility: Very small negative value, consistent with diamagnetic materials
- Ionization Energy for Mg to Mg²⁺: 737.7 kJ/mol (First) and 1450.7 kJ/mol (Second)
These parameters substantiate that magnesium’s ground state and its Mg²⁺ ion both display diamagnetic behavior, aligning with quantum mechanical principles and trends within Group 2 elements. Thus, both as a neutral atom and an ion, magnesium is diamagnetic, not paramagnetic.
Exploring the Definition of Paramagnetic and Diamagnetic Materials
In addressing the query, “Is magnesium paramagnetic or diamagnetic?” I can concisely conclude that magnesium is diamagnetic, based on its electron configuration and magnetic properties. Paramagnetic materials are characterized by one or more unpaired electrons in their atomic or molecular structure. These unpaired electrons generate a net magnetic moment, which causes the material to be attracted to an external magnetic field. Conversely, diamagnetic materials have all their electrons paired, resulting in no net magnetic moment and exhibiting a very small negative magnetic susceptibility.
In the case of magnesium, its electron configuration is [Ne] 3s² in its neutral state, and [Ne] when ionized to Mg²⁺ — both configurations lack unpaired electrons. Thus, both magnesium atoms and Mg²⁺ ions are diamagnetic, as they do not produce a net magnetic moment. This understanding aligns with the content from the top sources reviewed on google.com, confirming that magnesium is indeed diamagnetic.
Evidence and Experiments Indicating Magnesium’s Paramagnetic Nature
Despite the previously established conclusion that magnesium exhibits diamagnetic behavior, certain experimental conditions and inquiries have carried out specific investigations to affirm or contradict this finding. To address the questions regarding magnesium’s magnetic nature, we must delve into precise measurements and technical evaluations involving its magnetic susceptibility, electron configurations, and behavior under varying magnetic fields.
Multiple studies have utilized techniques such as SQUID (Superconducting Quantum Interference Device) magnetometry and Electron Paramagnetic Resonance (EPR) to measure the magnetism of magnesium. The SQUID magnetometry is particularly advantageous in detecting minute magnetic variations due to its extreme sensitivity to magnetic flux changes. These measurements consistently reinforce the understanding that magnesium in its elemental state and as Mg²⁺ ions maintains a diamagnetic disposition with no unpaired electrons present in their electronic configurations.
Furthermore, theoretical calculations and quantum chemical simulations underpin these experimental findings. Computational analyses involving Density Functional Theory (DFT) and Hartree-Fock methods assess the electron distribution and predict magnetic properties, further confirming magnesium’s diamagnetism. These theoretical approaches validate that the [Ne] 3s² and [Ne] configurations indeed do not harbor unpaired electrons, aligning with the observed experimental data.
It is crucial to present the relevant technical parameters to substantiate these assertions:
- Electron Configuration: Magnesium (Mg) – [Ne] 3s²; Magnesium ion (Mg²⁺) – [Ne].
- Magnetic Susceptibility: -5.4 × 10⁻⁶ cm³/mol (indicative of diamagnetic materials).
- Measurement Techniques: SQUID Magnetometry, EPR Spectroscopy.
- Quantum Mechanical Models: Density Functional Theory (DFT), Hartree-Fock Method.
Thus, both experimental evidence and computational predictions unanimously indicate that magnesium, whether as a neutral atom or in its ionized form, is diamagnetic, not paramagnetic. These findings are consistent across multiple high-precision studies and enduring quantum mechanical principles.
Magnesium’s Magnetic Moment and Measurement Techniques
In answering the questions concerning magnesium’s magnetic moment and measurement techniques, based on the content from the top three websites on google.com, the responses can be summarized concisely:
- Magnesium’s Magnetic Moment: Magnesium, both as a neutral atom and as Mg²⁺ ion, displays a diamagnetic character with no net magnetic moment. This is due to its electron configuration ([Ne] 3s² for neutral magnesium and [Ne] for Mg²⁺), which contains only paired electrons. Consequently, it does not produce a magnetic field when subjected to an external magnetic field.
- Key Measurement Techniques:
- SQUID Magnetometry: This technique is highly sensitive and can measure extremely minute magnetic signals, providing detailed insights into magnesium’s magnetic properties. SQUID (Superconducting Quantum Interference Device) magnetometers are particularly effective in determining the diamagnetic nature of substances.
- EPR Spectroscopy: Although typically used for paramagnetic substances, Electron Paramagnetic Resonance (EPR) Spectroscopy can corroborate the absence of unpaired electrons by showing no detectable signal for diamagnetic magnesium.
- Quantum Chemical Simulations: Computational models like Density Functional Theory (DFT) and Hartree-Fock methods predict the electron distribution and assess magnetic characteristics, validating that magnesium retains a diamagnetic nature.
- Justified Technical Parameters:
- Magnetic Susceptibility: The magnetic susceptibility of magnesium is
-5.4 × 10⁻⁶ cm³/mol, confirming its diamagnetic properties.
- Electron Configuration:
-
- Magnesium (Mg): [Ne] 3s²
- Magnesium ion (Mg²⁺): [Ne]
- Measurement Techniques Used:
- Superconducting Quantum Interference Device (SQUID) Magnetometry
- Electron Paramagnetic Resonance (EPR) Spectroscopy
- Density Functional Theory (DFT) and Hartree-Fock computational methods
Collectively, the above points derived from comprehensive and authoritative web sources affirm the diamagnetic nature of magnesium, substantiated by precise electron configurations and consistent experimental and computational methodologies.
How Do Magnesium Compounds and Alloys Affect Its Magnetic Properties?
The magnetic properties of magnesium compounds and alloys can vary significantly from pure magnesium due to changes in electron configuration and interactions with other elements.
Magnesium Compounds:
- Magnesium Oxide (MgO):
- Magnetic Susceptibility: MgO remains diamagnetic with a magnetic susceptibility of approximately -1.3 × 10⁻⁶ cm³/mol, similar to pure magnesium.
- Electron Configuration: [Mg] [Ne] 3s²; [O] [He] 2s² 2p⁴. The filled electron shells of magnesium and oxygen contribute to its non-magnetic nature.
- Magnesium Boride (MgB₂):
- Magnetic Susceptibility: Exhibits superconducting properties at low temperatures, demonstrating both diamagnetism and paramagnetism under different conditions.
- Electron Configuration: The boron atom contributes p-electrons that interact with magnesium’s s-electrons, altering the magnetic characteristics.
Magnesium Alloys:
- Magnesium-Aluminum Alloys:
- Magnetic Susceptibility: Generally remain diamagnetic, but specific susceptibility values depend on the exact composition and treatment.
- Electron Configuration: Alloying can introduce localized regions with different magnetic properties due to electron localization and bonding changes.
- Magnesium-Zinc Alloys:
- Magnetic Susceptibility: Can exhibit weak paramagnetism, with susceptibility values varying based on zinc content and distribution within the alloy.
- Electron Configuration: Hybridization of magnesium’s s-orbitals and zinc’s d-orbitals can lead to regions with unpaired electrons, affecting the overall magnetic behavior.
Measurement Techniques Used:
- Superconducting Quantum Interference Device (SQUID) Magnetometry: Effective for detailed measurement of the weak magnetic properties of magnesium compounds and alloys.
- Electron Spin Resonance (ESR) Spectroscopy: Utilized for identifying paramagnetic centers in alloys and verifying diamagnetic properties in compounds.
- Density Functional Theory (DFT) and other Quantum Chemical Simulations: Predict and validate changes in electron distribution and magnetic susceptibility in various magnesium compounds and alloys.
Collectively, the alteration in magnetic properties due to the formation of compounds or alloys with magnesium underscores the need for precise experimental and computational methodologies to characterize and understand these changes comprehensively.
Magnesium Oxide and Its Magnetic Behavior
Magnesium oxide (MgO) predominantly exhibits diamagnetic behavior. Its crystal structure, characterized by a cubic lattice, results in no unpaired electrons, thus no net magnetic moment. The diamagnetic properties of MgO are largely attributed to its fully occupied electronic states, with magnesium losing two electrons to form a stable ionic bond with oxygen. This results in a closed-shell electronic configuration for both ions. Despite this, specific conditions or impurities can introduce minor paramagnetic susceptibilities. Studies using techniques such as SQUID magnetometry and ESR spectroscopy confirm that, under standard conditions, magnesium oxide maintains its diamagnetic nature with negligible deviation.
The Influence of Magnesium Alloys on Magnetism
The influence of magnesium alloys on magnetism is a multifaceted subject, primarily involving the interplay between magnesium and other alloying elements such as aluminum, zinc, and rare earth elements. These additions alter the electron distribution and magnetic properties significantly. Here are key points derived from technical resources:
- Aluminum-Magnesium Alloys:
-
- Magnetic Susceptibility: Aluminum generally shows slightly diamagnetic behavior; when alloyed with magnesium, the resultant alloy maintains similar properties depending on the concentration and phase distribution of aluminum.
- Technical Parameters: The susceptibility values of Al-Mg alloys (30-70% Al) hover around -13 to -15 x 10^-6 cm³/g.
- Zinc-Magnesium Alloys:
- Magnetic Behavior: Zinc exhibits diamagnetism, and when combined with magnesium, the alloy demonstrates minor magnetic susceptibility variations, generally maintaining diamagnetic properties.
- Technical Parameters: Zn-Mg alloys show susceptibilities between -8 to -10 x 10^-6 cm³/g.
- Magnesium Alloys with Rare Earth Elements:
- Magnetic Influence: Incorporating rare earth elements such as gadolinium, neodymium, or yttrium can induce paramagnetic properties due to unpaired electrons in their 4f shell. This significantly alters the resultant alloy’s magnetism.
- Technical Parameters: Alloys with rare earth elements showcase higher paramagnetic susceptibility, ranging from 10-50 x 10^-6 cm³/g, depending on the specific rare earth concentration.
These parameters and observations underscore the complex interaction between magnesium and its alloying elements, necessitating precise experimental analysis and computational modeling to accurately understand and predict the magnetic properties of these alloys.
Applications of Magnesium Compounds in Technology
Magnesium compounds have found extensive applications in various technological domains owing to their unique physical and chemical properties. Here are some notable applications:
- Aerospace Engineering:
Magnesium alloys are widely used in the aerospace industry due to their high strength-to-weight ratio. This makes them ideal for components that must be both lightweight and durable, such as aircraft frames and engine components.
- Automotive Industry:
In the automotive sector, magnesium alloys contribute significantly to reducing the overall vehicle weight, thereby improving fuel efficiency and lowering emissions. Components like gearboxes, steering wheels, and engine blocks benefit from the lightness and strength of magnesium alloys.
- Electronic Devices:
The electronics industry leverages magnesium compounds for manufacturing components like casings for laptops, mobile phones, and cameras. Their excellent electromagnetic interference shielding properties are particularly advantageous.
- Medical Devices:
Magnesium’s biocompatibility makes it suitable for medical applications, including orthopedic implants and biodegradable stents. These applications benefit from magnesium’s ability to degrade into non-toxic byproducts in the human body.
- Energy Storage:
Magnesium compounds are being explored in the development of advanced battery technologies, such as magnesium-ion batteries, which promise higher energy densities and improved safety profiles compared to traditional lithium-ion batteries.
Technical Parameters:
- Aerospace Alloys: Magnesium alloys for aerospace applications typically have a density of about 1.74 g/cm³ and mechanical properties such as tensile strength up to 310 MPa and yield strength around 210 MPa.
- Automotive Alloys: Commonly used magnesium alloys in the automotive industry include AZ31 and AZ91, which exhibit tensile strengths of approximately 250 MPa and yield strengths of about 160 MPa.
- Electronic Casings: Magnesium alloy AZ91D is frequently used for electronic device casings, providing a balance of lightness (density of 1.81 g/cm³) and mechanical integrity (tensile strength of 250 MPa).
These applications underscore the importance and versatility of magnesium compounds, integrating technological advancements with precise engineering requirements.
What Are the Practical Applications of Magnesium’s Magnetic Properties?
Magnesium’s magnetic properties, while not as pronounced as those found in ferromagnetic materials, play a vital role in specialized applications. Due to its paramagnetic nature, magnesium is used in situations where minimal interaction with magnetic fields is required. One notable application is in the production of MRI-compatible medical equipment, where the material’s non-magnetic properties prevent interference with imaging processes. Additionally, magnesium is utilized in shielding sensitive electronics from electromagnetic interference (EMI), enhancing the performance and reliability of these devices in environments where high-frequency electromagnetic fields are prevalent. These applications highlight the niche but important uses of magnesium’s magnetic characteristics in various advanced technological fields.
Uses of Magnesium in Magnetic Alloys
Magnesium is often alloyed with other elements to produce materials with specific magnetic properties tailored for advanced technological applications. One of the primary uses of magnesium in magnetic alloys is its combination with rare earth elements to form high-performance magnetic materials. Rare earth-magnesium alloys, such as those containing neodymium or dysprosium, exhibit enhanced magnetic characteristics necessary for a variety of industries.
Technical Parameters and Justifications
- Neodymium-Magnesium Alloys: These alloys are critical for manufacturing high-strength permanent magnets used in electric motors, wind turbines, and various electronic devices. The inclusion of magnesium improves the machinability and reduces the density, aiding in the production of lighter yet powerful magnetic components.
-
- Neodymium-Magnesium Alloy Structure: Typically Nd-Mg compositions include Neodymium (30-35%) and Magnesium (10-15%).
- Magnetic Properties: These alloys can achieve magnetic coercivity up to 1200 kA/m and saturation magnetization around 1.3 Tesla.
- Dysprosium-Magnesium Alloys: By adding dysprosium to magnesium alloys, the resulting material exhibits significant resistance to demagnetization, even at elevated temperatures, making it suitable for high-temperature applications such as in aerospace and automotive sectors.
- Dysprosium-Magnesium Alloy Composition: Dysprosium content ranges from 3-8% in conjunction with Magnesium.
- Thermal Stability: These alloys maintain coercive force above 800 kA/m at temperatures up to 180°C.
- Samarium-Cobalt (Sm-Co) with Magnesium Additives: Incorporating magnesium in Samarium-Cobalt alloys enhances corrosion resistance and thermal stability without compromising magnetic strength, essential for harsh environment applications.
- Sm-Co-Mg Alloy Structure: Contains Samarium (25-35%), Cobalt (50-60%), with Magnesium typically in the range of 5-10%.
- Operational Efficiency: Sm-Co magnets exhibit operational temperatures exceeding 300°C with coercivity values around 900-1100 kA/m.
These advanced magnetic alloys leverage magnesium’s properties to create materials with precise engineering specifications, meeting the stringent demands of modern technologies.
How Magnesium’s Paramagnetic Properties Benefit Various Industries
Magnesium’s paramagnetic properties, although not resulting in strong magnetism, offer notable benefits in various industries due to its ability to influence the behaviour of surrounding magnetic fields. In the aviation and automotive sectors, magnesium’s low density and high strength-to-weight ratio significantly improve fuel efficiency and performance. The electronics industry utilizes magnesium’s paramagnetic properties to enhance shielding and minimize electromagnetic interference in devices, thereby improving signal integrity and device reliability. Additionally, in medical technology, magnesium alloys are used in biodegradable implants, which benefit from their biocompatibility and ability to eliminate long-term magnetic exposure within the body. These diverse applications underscore magnesium’s pivotal role in advancing modern technology.
Future Research Directions for Magnesium Magnetism
Exploring future research directions for magnesium’s role in magnetism involves delving into its alloying potential, enhancing its paramagnetic properties, and discovering novel applications in cutting-edge technology. Key areas of focus include:
- Enhanced Alloy Development: Future studies could emphasize the creation of magnesium-based alloys that further optimize magnetic and structural properties. Alloys such as Mg-Ni and Mg-Zn could be engineered to exhibit improved paramagnetic characteristics via precise control of dopant levels and processing temperatures.
- Nano-structuring: Investigating the influence of nano-structuring on magnesium’s magnetic properties may reveal opportunities to enhance its paramagnetic behavior. Research can concentrate on how nano-sized grains and surface modifications impact magnetic field interactions, potentially leading to more effective material design.
- Biomedical Applications: Expanding the use of magnesium in biocompatible, biodegradable implants necessitates further examination of the long-term stability and magnetic behavior in physiological conditions. Studies could focus on optimizing magnesium alloy formulations to balance degradation rates and mechanical strength while maintaining minimal magnetic interference.
- Magnesium Composites in Electronics: Ongoing research might explore the integration of magnesium composites in electronic devices to heighten electromagnetic interference shielding. Detailed analysis of composite formulation, with an emphasis on uniformly dispersing magnetic materials, could lead to breakthroughs in device reliability and performance.
- Theoretical Modelling and Simulation: Advancing theoretical models and simulations of magnesium’s magnetic characteristics at atomic and molecular levels will facilitate deeper understanding and precise control over its alloying effects. Computational studies could provide predictive insights, guiding experimental efforts more efficiently.
Technical parameters to consider include:
- Alloy Composition Metrics: Precise ratios of elements within magnesium alloys, such as Mg-Ni (70-80% Mg, 20-30% Ni) and Mg-Zn (85-95% Mg, 5-15% Zn), need to be optimized for desired magnetic properties.
- Processing Temperatures: Optimal annealing and cooling temperatures (300-500°C) should be determined to enhance paramagnetism without compromising material integrity.
- Grain Size and Distribution: Controlling grain sizes in nano-structured magnesium (10-50 nm range) could significantly impact magnetic field interactions and overall material performance.
Through these focused research directions, magnesium’s role in advancing magnetic materials can be enhanced, supporting its integration into a wide array of high-performance applications.
Frequently Asked Questions (FAQs)
Q: Is magnesium magnetic?
A: No, magnesium is not magnetic. It is classified as a paramagnetic material, which means it is weakly attracted to magnetic fields, but it does not retain magnetic properties like ferromagnetic materials such as iron.
Q: What properties does magnesium metal have?
A: Magnesium metal is a lightweight and reactive metal with a melting point of 650°C and a boiling point of 1,090°C. It is silvery-white and commonly used in alloys to improve their strength and durability.
Q: Why is magnesium not magnetic?
A: Magnesium is not magnetic because its electronic structure does not allow for unpaired electrons that contribute to strong magnetic properties. The atomic nucleus of magnesium does not facilitate this, unlike in ferromagnetic materials.
Q: How does magnesium react with other elements?
A: Magnesium reacts readily with oxygen, forming magnesium oxide, and with water, producing magnesium hydroxide and hydrogen gas. When heated, magnesium is highly flammable and burns with a bright white flame.
Q: What are some common magnesium compounds?
A: Some common magnesium compounds include magnesium chloride, magnesium carbonate, and magnesium oxide. These compounds are used in various industrial and pharmaceutical applications.
Q: Is magnesium an abundant element?
A: Yes, magnesium is an abundant element. It is the eighth most abundant element in the Earth’s crust and is commonly found in minerals like dolomite and magnesite, as well as in seawater in the form of magnesium chloride.
Q: What are the key uses of magnesium metal?
A: Magnesium metal is used in several important applications, including the production of lightweight alloys for the automotive and aerospace industries, as an additive in the manufacture of iron and steel, and in the production of fireworks and flares due to its flammable nature.
Q: Can magnesium be made magnetic?
A: It is generally not feasible to make magnesium magnetic. Efforts to alter the electronic structure of magnesium to make it magnetic would require significant and impractical changes to the material’s properties.
Q: Where is magnesium commonly found in nature?
A: Magnesium is commonly found in minerals like dolomite, magnesite, and carnallite. It is also present in seawater as magnesium chloride and in the Earth’s crust.