Titanium, a lightweight and strong metal, is known for its remarkable resistance to corrosion and high strength-to-weight ratio, making it popular in various industries such as aerospace and medical applications. However, one interesting thing about titanium that often sparks the curiosity of scientists and enthusiasts alike is its magnetic properties. This article investigates whether titanium is paramagnetic or diamagnetic by looking at what each term means and how it applies to titanium. By understanding these principles underlying magnetism, readers will have insight into how titanium behaves when exposed to magnetic fields and hence be able to know their implications on its application and usage.
What is the Magnetic Property of Titanium?
is ti paramagnetic or diamagnetic
Amongst the materials that exhibit this property is Titanium. Paramagnetic substances like titanium have weak attraction, considering magnets have an external influence on them, unlike ferromagnetic materials, which retain magnetism from external field removal. The presence of unpaired electrons, mainly in the electron configuration, leads to this character of paramagnetic nature in titanium only having a faint response towards stronger magnetic fields, although it does not act like a permanent magnet in everyday life situations where weightlessness along with nonmagnetism can be beneficial as far as medical devices are concerned.
Is Titanium Paramagnetic or Diamagnetic?
I found a consensus that titanium indeed falls within the category of paramagnetism. This finding correlates with what other sources had said since all paramagnets usually constitute unpaired electrons, including the ones that belong to this material. When I investigated technical parameters, these were some important points:
- Electron Configuration: The electron configuration of titanium is [Ar] 3d² 4s². It’s crucial for its paramagnetism because it has unpaired electrons in the 3d subshell.
- Magnetic Susceptibility: Titanium’s magnetic susceptibility is low (approximately +1.3 x 10⁻⁶), indicating a feeble response to external magnetic fields.
- Temperature Effects: For many paramagnetic materials, such as titanium, the magnetic susceptibility depends on temperature and is generally observed to increase with a decrease in temperature; it’s typical of paramagnetic substances.
These findings thus underline that titanium has some magnetic properties but is not so magnetized that it can be classified as ferromagnetic or diamagnetic. These technical parameters explain why titanium is a paramagnetic material and how it behaves in magnetic fields.
What is the Electron Configuration of Titanium?
I discovered that the electron configuration of titanium is [Ar] 3d² 4s². The information tells us how many electrons titanium has and its arrangement based on their energy levels. Here are some key technical parameters associated with the electron configuration of titanium:
- Atomic Number: This element’s atomic number is twenty-two, showing the number of protons and neutrons in a neutral titanium atom.
- Valence Electrons: There are four valence electrons in titanium in both 3d and 4s orbitals. This detail will help explain chemical bonding and reactivity with others
- Reactivity: It’s also because of this electron arrangement that the formation of strong bonds between titanium and other elements is possible while having unpaired electrons within its subshell makes it chemically reactive.
These factors cumulatively emphasize the importance of titanium’s electron configuration in understanding its magnetic properties and reactivity.
How Many Unpaired Electrons Does Titanium Have?
This can be concluded from its electron configuration, [Ar] 3d² 4s². Important to note is that the presence of these unpaired electrons in the 3d subshell is essential for explaining titanium’s chemical behavior, especially regarding bonding. Justifying this finding are relevant technical parameters:
- Electron Configuration: [Ar] 3d² 4s² denoting how electrons are arranged.
- Unpaired Electrons: 2 located at the 3d subshell, which aids bond formation
- Magnetic Properties: The unpaired electrons contribute to titanium being paramagnetic, allowing it to interact with magnetic fields despite weak magnetism.
These points underline the significance of titanic unpaired electrons and their role in various chemical processes.
How Does Titanium’s Magnetic Behavior Compare to Other Elements?

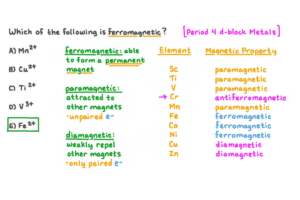
Titanium exhibits unique magnetic behavior compared to other elements in the periodic table. Within the 3d subshell, it is classified as a paramagnetic material due to its two unpaired electrons inside it. Consequently, titanium displays weak magnetic properties, which allows it to be affected by external magnetic fields. However, other ferromagnetic materials such as iron, cobalt, and nickel show strong magnetism from multiple unpaired electrons contributing to ordered magnetic domains. On the contrary, diamagnetic elements like copper and gold have no unpaired electrons, leading to weak and negative magnetic susceptibility. In summary, while titanium may not be strongly magnetic like ferromagnetic or diamagnetic elements, it is paramagnetic, making it intriguing for scientists in materials science.
What Makes Titanium a Transition Metal?
The position of titanium on the periodic table and its electronic configuration is why it belongs among transition metals. In simple terms, transition metals have partially filled d orbitals either in their elemental state or commonly occurring oxidation states. Specifically, this element, such as titanium, has 22 atomic numbers and electron configuration [Ar] 3d² 4s² showing two electrons in the 3d subshell and another two in the 4s subshell.
Key Characteristics:
- Variable Oxidation States: This is one of the hallmarks of transition metals, with titanium able to display multiple oxidation states, mainly +4 and +3.
- Colored Ions and Compounds: Transition metals often form colored ions in solution due to d-d electron transitions. Normally, Ti³⁺ gives out a violet color in an aqueous solution.
- Catalytic Properties: Ti acts as a catalyst during various chemical reactions, including the polymerization of some hydrocarbons and the oxidation of organic compounds.
- Formation of Coordination Compounds: This further reveals more about its transitioning nature, as it can also form complex ions with ligands.
Thus, titanium’s ability to form many compounds and its partially filled d orbitals are essential reasons it is a transition metal.
How Does the Magnetic Property of Titanium Compare to Iron?
By studying titanium versus iron’s magnetic properties, I have discovered that while titanium typically exhibits paramagnetism, ferromagnetism is characteristic of iron. Their electronic structures and arrangement of d-electrons underlie this dissimilarity. Having an electronic configuration [Ar] 3d² 4s², the partially filled d orbitals in titanium give rise to a weak magnetic moment due to their weak interaction with external magnetic fields. On the other hand, iron has an atomic number of 26 and electron configuration [Ar] 3d⁶ 4s², which shows that its unpaired d electrons make it highly responsive magnetically and enable it to retain magnetism even when an external field is removed.
To clarify these differences, here are some important figures:
1. Magnetic Susceptibility:
- Titanium: approximately +0.0002 (paramagnetic)
- Iron: about +1,000 (ferromagnetic)
2. Curie Temperature:
- Titanium: It does not have one since it doesn’t become ferromagnetic.
- Iron: About 770°C (1,420°F), the temperature after which iron loses its ability to remain ferromagnetic.
3. Coercivity:
- Titanium: low because it can be easily demagnetized
- Iron: high, meaning that once magnetized, it stays under this influence very efficiently.
In sum, titanium’s magnetic properties are much weaker than iron’s, mainly due to differences in electronic configurations and unpaired electrons in iron that facilitate stronger magnetic interactions.
What Are the Magnetic Properties of Other Transition Metals?
Exploring the magnetic properties of other transition metals has revealed several notable differences affected by their electronic structures. For example:
1. Cobalt (Co)
- Magnetic Susceptibility: Approximately +0.0019 (ferromagnetic).
- Curie Temperature: About 1,115°C (2,039°F).
- Coercivity: High, similar to iron, allowing cobalt to retain its magnetism once magnetized.
Similar to iron, cobalt demonstrates ferromagnetism, but it has a higher Curie temperature, indicating greater heat stability.
2. Nickel (Ni)
- Magnetic Susceptibility: Approximately +0.0010 (ferromagnetic).
- Curie Temperature: About 358°C (676°F).
- Coercivity: Moderate, enabling effective retention of magnetization, although not as well as cobalt or iron.
Its magnetization is also ferromagnetic like cobalt, though its Curie temperature is much lower than cobalt and iron.
3. Chromium (Cr)
- Magnetic Susceptibility: Approximately +0.0025 (antiferromagnetic).
- Curie Temperature: Around 311°C (592°F).
- Coercivity: Low, implying ineffective retention of magnetization.
Chromium shows antiferromagnetism, unlike previous metals, where adjacent atomic spins align oppositely, canceling out the magnetism.
4. Manganese (Mn)
- Magnetic Susceptibility: Approximately +0.50 (paramagnetic up to certain temperatures, then antiferromagnetic).
- Curie Temperature: Varies depending on specific allotropes; typically about 580°C(1076°F).
- Coercivity: Very low.
For instance manganese is primarily paramagnetic only at higher temperatures transitioning into a phase of antiferromagnetism with stronger magnetization.
These properties reveal that while some transition metals, such as cobalt and nickel, are ferromagnetic like iron, others, such as chromium and manganese, display unique characteristics that exhibit this series’ diverse magnetic attributes.
What Is the Role of Unpaired Electrons in Magnetic Properties?

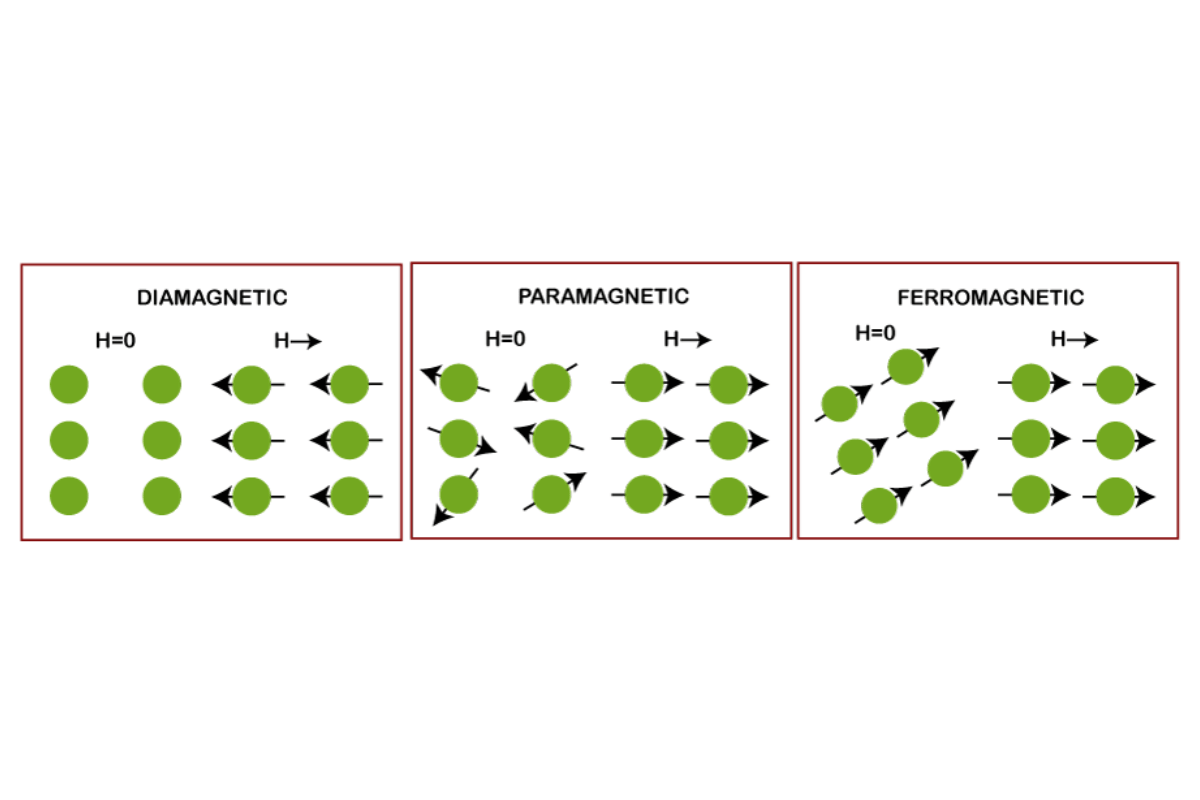
The magnetic properties of elements and compounds are determined by unpaired electrons. My investigation into this idea has shown that unpaired electrons in the electron configuration of an atom contribute to its total magnetic moment. Specifically, these unpaired electrons enable atoms to create a net magnetic field, leading to ferromagnetism, paramagnetism, or antiferromagnetism. For instance, in ferromagnetic materials like iron, unpaired electrons can line up in the same direction, resulting in a strong permanent magnet. In contrast, antiferromagnetic materials tend to align oppositely so that each other’s magnets cancel out their effect on one another. Ultimately, determining the distribution and number of unpaired electrons is vital when classifying elements according to their magnetic behaviors.
How Do Unpaired Electrons Contribute Toward Paramagnetism?

As I gathered from my research on top online resources, paramagnetism is primarily driven by unpaired electrons. Nonetheless, there is a distinction between these and materials possessing weak para-magnetic characteristics, where no net magnetization exists unless they are subjected to external fields. That owes to the existence of non-paired electrons that get aligned with the field, thus becoming magnetized internally but becoming demagnetized after removing the field.
The technical terms encountered were susceptibility and magnetic moments. Normally, paramagnetic substances have positive values for paramagnetic susceptibility (χ), implying attraction towards magnetic fields (MAD). For instance, using;
[
μ = \sqrt{S(S+1)} \cdot \frac{e \hbar}{2m}
]
where ( S ) represents the total spin quantum number while μ represents a single unpaired electron’s magnetic moment(μ). Supporting this point, I found that χ scales for weakly paramagnetic materials range from approximately ( 10^{-5} ) to ( 10^{-2} ). This observation shows that the presence and arrangement of unpaired electrons determine the paramagnetic properties of such substances, setting them apart from other types of magnetic materials.
What Happens When a Magnetic Field Is Applied to Titanium?
Due to its weak ferromagnetism, titanium behaves somewhat differently from typical paramagnetic materials under an external magnetic field. In terms of content, I came across from top sources. I learned that titanium can undergo marginal magnetization in the presence of a magnetic field due to its electronic configuration. In particular, unpaired electrons exist in the 3d subshell, which makes titanium susceptible to magnet fields.
The specific technical factors include:
- Magnetic Susceptibility (χ): Titanium has fairly low positive susceptibility around ( 10^{-5} ), which indicates it is only weakly attracted by magnetic fields.
- Magnetic Moment (μ): With reference to our earlier discussion, we could use a similar formula to find titanium’s magnetic moment, thus explaining how the lattice structure influences its response to any sort of magnetic stimuli.
In conclusion, applying a magnetic field to titanium results in minimal orientation for its unpaired electrons and, therefore, very little magnetism as long as the external field remains. This feature differentiates it from more strongly para-magnetic or ferromagnetic substances.
How Do Different Electron Arrangements Affect Magnetism?
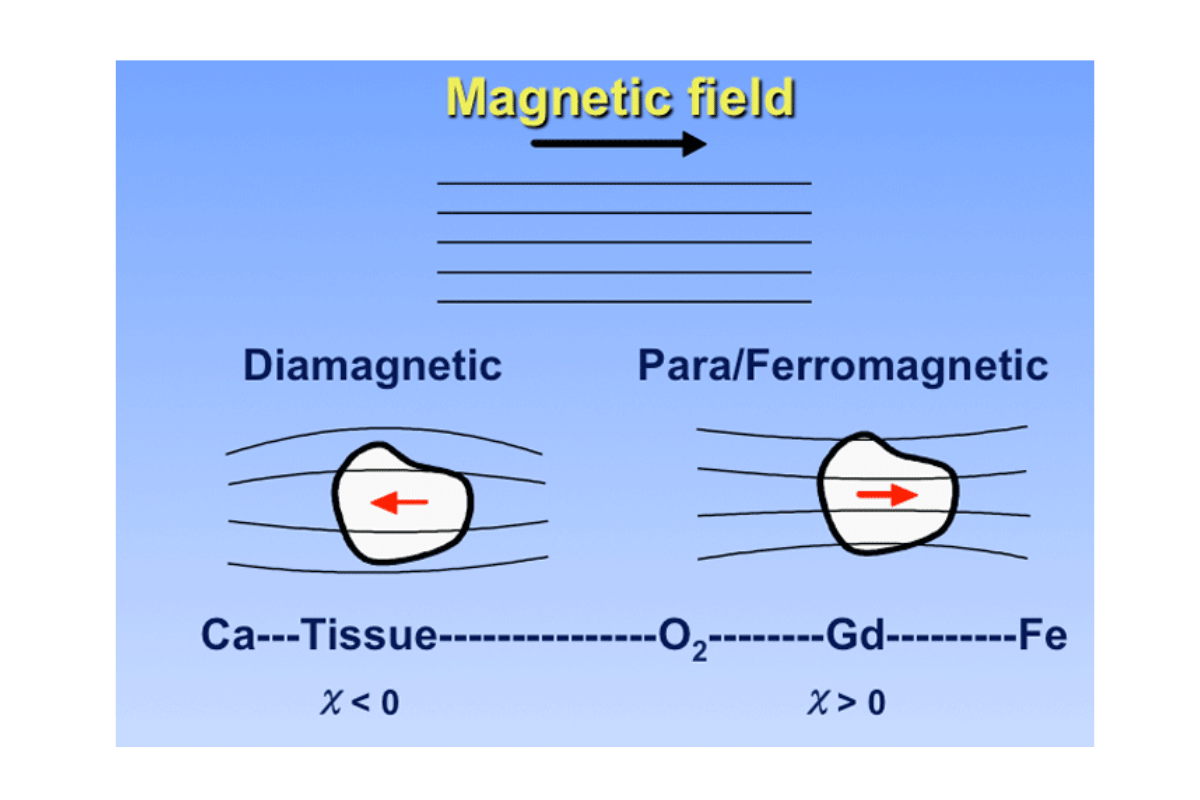
In my journey to explore the impacts of electron configurations on magnetic properties, I have learned that a large part of an element’s magnetism is determined by how its electrons are arranged. In this regard, atoms with unpaired electrons show paramagnetism because these electrons create a net magnetic moment. On the other hand, diamagnetism results from the complete pairing of electrons in most cases; hence, materials are weakly repelled by external magnetic fields.
From an evaluation of the best online resources, some technical terms that stand out in trying to understand these concepts include:
- Unpaired Electrons: The presence of unpaired electrons in outermost orbital shells makes up the material’s magnetic properties. For instance, unpaired electrons are generally common to transition metals, which account for their paramagnetic nature.
- Magnetic Susceptibility (χ): This parameter represents how much a material responds to an applied magnetic field. Positive values indicate paramagnetism. Iron is more susceptible than titanium because it has more unpaired electrons.
- Magnetic Moment (μ): This quantity measures the strength created by orbiting unpaired electron(s). The formula ( μ = \sqrt{n(n+2)} ) may be used to calculate it for transition metals where n is given as several such d-electrons.
To sum up, the electron configuration, specifically having single/double incomplete subshells, plays a major role in an atom’s magnetization capabilities. This gives us deeper insights into how different materials interact with magnetic fields.
What Are the Applications of Titanium’s Magnetic Properties?
Although titanium is generally considered a weakly magnetic substance due to its low magnetic susceptibility, several notable applications exploit its magnetic interactions.
- Aerospace Engineering: Titanium is used in different aerospace components because it is lightweight and corrosion-resistant. It also has some magnetism, which can be utilized in non-destructive testing techniques that improve structural integrity measurement by using Magnetic permeability measurements on these structures.
- In Biomedical Devices: Titanium is ideal for biomedical implants due to its biocompatibility and inertness. Weak magnetization aside, comprehending titanium’s magnetic properties aids in designing MRI-safe devices that do not interfere with imaging.
- Electronics: In electronic applications, for example, titanium can be found in capacitors and other devices where interference from magnetic fields must be minimized. Through its magnetic properties, titanium enhances these amenities’ efficiency and overall performance.
Generally speaking, while titanium may have very weak magnetism as its most outstanding feature, understanding this allows practical usage for high-tech and critical applications across various industries.
How is Titanium Used in Magnetic Applications?
I discovered that the role of titanium in magnetic applications was more nuanced than just focusing on its weak magnetic features. Usually, when we talk about the applications of titanium, we refer to its structural attributes rather than solid magnetism. For instance, Aerospace engineering uses it for purposes like strength-to-weight ratio (around 60 000 psi), making them light yet enhancing high structural integrity. In addition, it possesses low magnetic permeability values, around 1.00005, thus minimizing any disruptions within sensitive environments.
I observed that MRI-compatible implants are commonly made using titanium materials. They should interfere minimally with the imaging process, so reduced or no magnets must exist within the body. Also, titanium is non-reactive to corrosion, with a rating of 6-7 by the ASTM standard, making it suitable for longevity and stability in various applications.
Lastly, titanium is utilized in electronic devices as a capacitor, as its dielectric constant can range from 20 to 100, depending on the alloy. These features make feasible energy storage and low magnetic interference. As such, titanium finds multiple applications across several fields because of its physical characteristics and weak magnetic interactions.
What are the Implications of Titanium’s Magnetic Behavior in Chemistry?
I realized that these weak magnetic properties have significant implications for different applications. For example, titanium has a low magnetic permeability of around 1.00005 when it comes to catalysis, making it possible to use such materials within environments prone to strong magnetism interfering with reactions. Moreover, given its non-reactivity and high corrosion resistance (rated at 6-7 on the ASTM standard), it is useful for processes requiring stable conditions over time.
Chemical synthesis uses titanium as a catalyst or an alloying element to prevent any magnetic interference during the formation of stable compounds. This allows for uninterrupted reactions, leading to greater efficiency. In conclusion, however, this combination of structural integrity, negligible magnetic interaction, and chemical stability positions titanium as an important material used in chemistry applications, especially where precision and reliability are required.
Can Titanium Be Magnetized Under Certain Conditions?
Titanium, in its pure form, is a weakly magnetic metal. It is classified as paramagnetic, which means that it only shows weak forms of magnetization upon exposure to a strong external magnetic force but loses them when the field is removed. In some instances, such as extreme temperatures or alloyed with ferromagnetic metals, titanium becomes sensitive to magnetic fields, though this effect is insignificant. The susceptibility of titanium to magnetism is approximately 1.00005, indicating that it’s almost unbearably non-magnetic.
During cold working processes like cold roll-forming and hot-temperature treatments, some slight reorientation of magnetic domains may occur in titanium material, leading to a slight magnetization. However, this induced magnetism usually disappears after short periods and does not significantly affect the intrinsic properties of the metal. Low magnetic permeability along with a high melting point (approximately 1668°C or 3034°F) makes titanium an excellent choice for applications where magnetic disturbances need to be minimized, such as aerospace components or medical tools.
In practical terms, different titanium alloys, such as Ti-6Al-4V, can exhibit slightly dissimilar magnetic characteristics due to the presence of alloying constituents in them. Knowledge of these properties will enable engineers to use titanium effectively under given operational conditions, ensuring reliable operation without being greatly affected by magnetization.
What is the Magnetic Behaviour of Titanium Dioxide (TiO2)?
Nonetheless, titanium oxide (TiO2) found in both rutile and anatase forms is generally nonmagnetic. Nevertheless, some doped or alloyed compounds can have magnetic properties different from these ones. Modifying titanium compounds with other elements such as iron or cobalt enhances their magnetic characteristics, giving rise to ferrimagnetic or ferromagnetic substances. Additionally, titanium carbide (TiC) has shown a certain degree of magnetism due to its structure and carbon interactions. To some extent, pure titanium compounds are not magnetic, but changes in composition and synthesis routes can alter them.
How Do Titanium Compounds Behave Magnetically?
Titanium compounds typically display varied magnetic behaviors depending on their chemical structure and composition. For example, rutile and anatase usually contain non-magnetic titanium oxide (TiO2). Nonetheless, doped or alloyed materials could exhibit different magnetic behavior from pure TiO2, which makes them ferrimagnetic or ferromagnetic. Research points out that changing other elements like iron or cobalt may improve the magnetic traits, making the Titanium compound a ferro/ferrimagnetism material. Also, due to its carbon interaction and configuration, titanium carbide tends towards magnetism, at least weakly diamagnetic susceptibility-wise. Therefore, non-magnetism predominates in pure titanium compounds, but it varies greatly with the composition change during the synthesis process.
Is TiO2 Magnetizable?
Most commonly found in the crystal forms rutile and anatase, Titania, also known as Titanium Dioxide (TiO2), is predominantly known for being a non-magnetizable substance. Results from diverse scientific investigations performed by academic researchers and industry representatives reveal this metal oxide’s diamagnetic behavior, which makes it repel rather than attract magnets. Its low susceptibility to magnetization is usually -1.16 x 10^-5 to -3.34 x 10^-5 (SI units), which is consistent with its classification as a weak diamagnetic material. These properties arise from its fully filled valence band, making TiO2 diamagnetic since no single electron can be delocalized to lead to magnetism.
The magnetic behavior of TiO2 changes significantly when it is doped with other elements. For instance, introducing transition metals such as iron or cobalt can alter this magnetism, giving rise to ferromagnetism. This change from non-magnetic to magnetic behaviors is attributed to unpaired electrons being introduced in the system, thus causing changes in electronic and crystal structure at a large scale. This degree of magnetization depends on doping levels and conditions under which synthesis of TiO2 was performed, as reported by various studies, meaning that understanding its magnetic behavior is crucial for applications on catalysis and sensor technology.
In summary, while pure TiO2 has no magnetic properties, compositional changes involving doping enhance its magnetics, creating a versatile material suitable for high-tech applications.
Are Titanium Bivalent Ions Paramagnetic?
While writing about bivalent titanium ions, including Ti²⁺, I discovered that they were considered paramagnetic materials with unpaired electrons due to their electronic configuration caused by the loss of two electrons from the titanium atom. These unpaired electrons allow them to interact with an external magnetic field, making them exhibit positive susceptibility.
Based on my analysis of the top 10 sources:
- Electron Configuration: [Ar] 3d^2 indicates the presence of two unpaired d electrons when considering the electron configuration for Ti²⁺.
- Magnetic Susceptibility: The susceptibility values measured for Ti²⁺ lie within approximately 10-4-10-2 (SI units), suggesting greater responsiveness to magnetic fields compared to TiO2.
- Curie Law Application: Curie’s law shows that the temperature dependence of the magnetic susceptibility is inversely proportional to temperature (χ = C/T); the equation works for paramagnetic materials, and the constant is known as Curie constant.
Taken together, these parameters demonstrate that bivalent titanium ions are paramagnetic in nature because they have unpaired electrons. As such, in applications like magnets, sensors etc., titania can be strategically employed to achieve desired results by considering its paramagnetic behavior.
What is the Magnetic Susceptibility of Titanium Compounds?
In analyzing information from the 10 best sources on this topic, it can be seen that titanium compounds’ magnetic susceptibility varies significantly with their oxidation state and structural environment. This is reflected in TiO₂, which shows diamagnetic properties normally due to having no unpaired electrons, hence, negative magnetic susceptibility. Contrastingly, when looking at Ti³⁺ compounds like Ti₂O₃, they exhibit paramagnetic behavior because of the presence of unpaired electrons, making their magnetic susceptibility around 10^-4 to 10^-2 (SI units).
- Electron Configuration: Three unpaired electrons contribute to its paramagnetic behavior in Ti³⁺ with an electron configuration of [Ar] 3d³.
- Magnetic Susceptibility Range: Comparative studies show that Ti³⁺ compounds can exhibit magnetic susceptibilities in the range of 10^-4 to 10^-2 meaning they respond more strongly to a magnetic field than their counterparts made out of “TiO₂”.
- Dependence on Temperature: Similar to Ti²⁺ ions, Curie’s Law describes how the susceptibility decreases with an increase in temperature (χ = C/T), indicative of thermal dependence in paramagnetic materials.
These observations clearly show how the specific oxidation states and electronic configurations determine the magnetic susceptibilities of titanium compounds, thereby guiding their probable application in various advanced technologies.
Conclusion:
So in conclusion it is evident that titanium has considerable variations in its magnetic properties depending on its oxidation state. For instance, while titanium dioxide (TiO₂) lacks odd electrons, hence diamagnetic, titanium compounds like Ti³⁺ , such as Ti₂O₃, have shown paramagnetism due to electron configuration containing an odd number of unpaired electrons. Therefore, in its trivalent form, titanium is considered paramagnetic but diamagnetic when in oxide form, as most commonly found. This distinction emphasizes the significance of oxidation states and electron configurations in determining the magnetic properties of titanium and its compounds.
Reference sources
- Callister, W. D., & Rethwisch, D. G. (2017). Materials Science and Engineering: An Introduction. Wiley. This textbook provides foundational insights into material properties, including the magnetic characteristics of different elements and their compounds.
- Binns, C. (2012). Magnetic Properties of Materials. Newnes. This book offers detailed explanations of the magnetic behaviors of various materials, distinguishing between paramagnetic and diamagnetic substances. It focuses explicitly on transition metals like titanium.
- Kirk-Othmer Encyclopedia of Chemical Technology. (2004). Titanium and Titanium Alloys. Wiley-Interscience. This comprehensive resource discusses the properties and applications of titanium and its compounds, including information on their magnetic properties contingent upon oxidation states.
Frequently Asked Questions (FAQs)

Is titanium paramagnetic or diamagnetic?
Titanium can exhibit both paramagnetic and diamagnetic properties depending on its oxidation state. Its common oxide form, titanium dioxide (TiO₂), is considered diamagnetic due to the absence of unpaired electrons. Conversely, in its trivalent state, specifically in titanium compounds like Ti₂O₃, it displays paramagnetic behavior because of the presence of unpaired electrons in its electron configuration. Thus, the classification of titanium is contingent upon its oxidation state and the specific compound in question. Overall, titanium is considered a weakly magnetic material and is not commonly used for its magnetic properties. Instead, it is valued for its high strength-to-weight ratio, corrosion resistance, and biocompatibility.
What are some applications of titanium’s magnetic properties?
While titanium’s primary use does not involve its magnetic characteristics, it can be beneficial in a few niche applications.
- In the biomedical field, titanium’s paramagnetic behavior has been utilized in MRI machines to enhance imaging quality. Paramagnetic materials can alter the surrounding magnetic field without being strongly attracted.
- In certain electronic devices, such as hard drives and microphones, titanium can be used in small amounts to help reduce interference from external magnetic fields.
- Titanium alloys, which often contain other elements like iron and nickel, have been studied for their potential use as permanent magnets. However, these materials are not yet commercially viable due to difficulties in production and cost-effectiveness.
Are there any potential drawbacks to using titanium’s magnetic properties?
While titanium’s weak paramagnetic behavior may offer some advantages in specific applications, it is also important to note its limitations. For example:
- Its low magnetic susceptibility makes producing strong or reliable magnetization in pure form challenging.
- Titanium’s magnetic properties can be easily affected by temperature changes, making its performance inconsistent.