Understanding how materials fail under stress is critical for the design of safe, durable structures in the field of material science and engineering. Loads on materials behave differently to brittle fracture mechanics which deals with the response of materials subjected to load until failure. Brittle and ductile fractures are two major types. These characteristics are evident in each type; this occurs differently based on them, and consequently engineered components’ performance and safety are significantly affected. This blog post attempts to distinguish between a ductile fracture and a brittle fracture, so that people can begin to have an understanding of material behavior when stressed. By investigating the inherent properties of materials as well as external elements that influence their crack modes, we intend to emphasize these concepts with practical significance spanning from everyday items like household items up to multifaceted engineering systems.
What is brittleness?
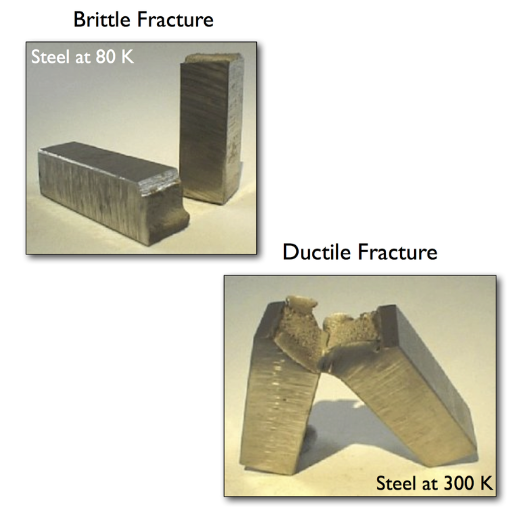
Image source: https://libatoms.github.io/
Brittleness is a quality of a substance that makes it break or crack without significant change in shape under pressure. Brittle materials undergo minimal plastic deformation and possess low energy absorption capacity before fracture. Glass and ceramics are two examples of brittle materials. They rupture quickly and disastrously, which makes them less suitable for use involving flexibility as well as resistance to impact. It is necessary to comprehend brittleness when selecting the right material for any engineering application depending on its performance requirements and safety demands.
How does a brittle material behave?
Materials that break at very little or no plastic deformation is what we call brittle materials; these materials usually fail suddenly once the stress surpasses their ultimate tensile strength. The capability of these items to absorb energy prior to breaking is almost zero. Thus, fracturing of many brittle materials occurs along planes normal to the applied tensile stress due to cleavage cuts in them. Furthermore, they lack ductility, so brittle fractures occur with almost no notice in many cases, hence this prudence by engineers when considering their behavior in engineering applications. Many times we find that they shatter or snap instead of bending or deforming.
Examples of brittle materials
Brittle materials which are:
- Glass: Commonly used in windows, bottles, and other containers, glass is a classic example of brittleness; it breaks into sharp pieces when hit.
- Ceramics: Used in everything from pottery to advanced engineering applications, ceramics have both hardness and fragility that makes them vulnerable to breaking under pressure.
- Concrete: Concrete is extensively used for construction purposes. But still it is brittle in tensile stress though strong in compression.
Other examples include cast iron, which can break easily under tension because of the presence of carbon, while some polymers and composite materials may be brittle depending on their individual chemical composition and treatment.
Why do brittle materials tend to fracture easily?
The limited ability to undergo plastic deformation before failure leads to easy fracture of brittle materials. This is because they lack ductility thus absorb little energy before breaking. Stress induced cracks travel fast normal to the force applied leading towards quick and sometimes unexpected fracturing. The microstructure of these materials consists of flaws such as micro-cracks or inclusions acting as points where stresses accumulate. These flaws can rapidly magnify and propagate within the load resulting abrupt failure of the material. In addition to this, atomic structure of the brittles lacks slip systems that exist in more ductile substances thus rendering them difficult to deform plastically hence prone to sudden rupture.
What is ductility?

Ductility, a material property that refers to the ability of a material to deform significantly before it fractures or ruptures, is often measured by the extent to which a sample can be drawn out into a wire when subjected to tension. Ductile materials unlike frail materials can absorb energy and deform considerably hence allowing them to take more stress without breaking. For instance, gold, silver and copper are some of the most common examples of ductile metals that can be drawn into thin wires. In many industrial applications, where materials must remain structurally sound under different loads and stresses, ductility is an important characteristic.
How is ductility defined in material science?
To be ductile is one of the main characteristics of a material in material science whereby it can undergo large plastic deformation without breaking. In this way, it is a mechanical property; which shows how soft or pliant a substance is under tension. Elongation and reduction of area are the two major indices used to evaluate ductility and they show the ability of a material to be deformed. Highly ductile materials tend to absorb and dissipate energy effectively, thus making them less brittle than those with low ductility. This feature is necessary for applications involving materials that must flex, twist or stretch without fracturing them thereby ensuring the durability and safety of products in many engineering and structural uses.
Characteristics of ductile materials
Ductile materials can experience large amounts of plastic deformation before breaking. They usually have high tensile strength and toughness, meaning that they can absorb a lot of energy without cracking. Such materials are easily made into wires or compressed into thin sheets by hammering. Ductile materials often show the yield point followed by a large strain hardening region, which increases their strength during deformation. Examples include copper, aluminum and steel which are highly malleable and can be worked at will so as to become flexible again.
Examples of ductile metals
Some of the metals commonly known for their ductility include:
- Copper: Copper is famously known because it has a good ductility where it can be stretched down to wires without rupture or hammered onto very thin sheets this makes it useful in electrical wiring and other industrial applications.
- Aluminum: It is also quite ductile like aluminum, which explains its use in making foils, cans as well as parts of airplanes among others since this metal easily takes on numerous forms while maintaining its strength and durability.
- Steel: The material is used heavily in construction, automotive industries and manufacturing especially low-carbon steels owing to their combination of strength and ductility. It finds broad application due to its ability to deform plastically without rupturing in various ways.
What is the difference between ductile and brittle materials?

Differences Between Ductile and Brittle Materials: The two types of materials differ mainly in how they react to stress and strain. Ductile materials like copper, aluminum, and steel can undergo substantial plastic deformation before breaking. During plastic deformation, a lot of energy is absorbed making it easy for the materials to be drawn into wires or beaten into sheets which are thin. Conversely, brittle materials such as glass and ceramics do not deform plastically before fracture but fail suddenly when subjected to stress. Fractures occur at much lower strains for brittle materials which usually have low energy absorption capacities thereby making them prone to abrupt failures that are catastrophic.
Key factors distinguishing ductility and brittleness
- Deformation Under Stress: When stressed, ductile materials can sustain significant deformation without rupturing so that they can be bent, twisted or stretched. On the other hand, brittle materials break almost immediately as they undergo little distortion prior to their fracturing.
- Energy Absorption: In the process of deformation, ductile materials absorb much energy allowing them to withstand higher impact or tensile loads. This property makes these types of material suitable for various structural applications. Contrarily, very less energy is absorbed by brittle material before its failure causing an immediate breakdown.
- Fracture Behavior: Generally speaking; in ductile materials, fracture occurs gradually with necking being observed while there is no conspicuous sign of plastic behavior exhibited by brittle ones hence fast fractures with sharp jagged surfaces.
- Atomic Structure and Bonding: Ductility or brittleness in a substance mainly depends on its atomic arrangement and bond strength. Metallic bonds found mostly in ductile substances allow atoms movement during stress conditions whereas ionic or covalent bonds often exist in rigid forms among many brittle ones discouraging movement leading to failure.
These criteria guide the selection of specific application-oriented manufacturing suited materials based on their mechanical properties under different conditions of service life.
Understanding the stress-strain curve
The stress-strain curve is a fundamental instrument of comprehending mechanical performance of materials under load. When stressed, material changes shape and this relationship between applied stresses and the resultant strains can be plotted to form the stress-strain curve. This curve gives invaluable insights into various stages of deformation in materials, which are divided into different sections as follows:
- Elastic Region: This indicates the area at the beginning of the graph where a material behaves elastically. The elastic region is such that if it were released from any pressure exerted on it, it would restore its original shape. The slope in this linear part is referred to as Young’s Modulus and it represents how stiff a given material is.
- Yield Point: Just beyond the elasticity region lies the yield point at which elastic deformation changes to plastic deformation. At this point permanent set occurs, and when taken off load will not return to its original configuration.
- Plastic Region: Material undergoes non-reversible deformation within this region. As stress continues to increase, the rate at which material stretches increases up until reaching its ultimate tensile strength.
- Ultimate Tensile Strength (UTS): It is whereby there appears a peak on a graph signifying maximum force that any material can withstand before yielding. Beyond this point, there will be necking resulting in reduction of section area.
- Fracture or Failure Point: It refers to when breaking or rupturing occurs since no more load can be carried by thematerials again. At this juncture, stress falls off sharply.
To predict how materials will behave under various loading conditions or select suitable engineering materials requires an understanding of what a stress-strain curve means about them.
Behavior under tensile stress
When tensile stress is applied to a substance, its conduct can be assessed with the help of the stress-strain curve so that its mechanical properties may be determined. The substance begins by deforming according to Hooke’s law up to its elastic limit. At this point in time, when the force is discontinued, the distortion reverts. The plastic stage starts after attaining maximum strain and signifies transition into plastic range. In this case, permanent distortion occurs because even if tensile load were relieved it will never go back to its original shape. With increasing tension acting on it the material gets more deformed until it undergoes ultimate tensile strength (UTS). Above UTS necking occurs leading to localized cross sectional area reduction that eventually results into failure point fracture. These phases help in predicting material behavior as well as ensuring appropriate materials for different engineering applications are chosen.
How do ductile and brittle fractures occur?

Ductile fractures are the result of a material being under significant plastic deformation before it fails. This kind of fracture is characterized by the development of a “neck” that is formed as a result of the decrease in cross-sectional area until the material eventually ruptures. Its fracture surface usually looks fibrous or dimpled, due to elongation and coalescence of micro-voids.
Contrarily, brittle fractures occur with some slight permanent deformation and propagate rapidly. They mostly take place when materials have little ductility and they are most common under high stress conditions. The granular or crystalline appearance seen on these surfaces indicates an abrupt and disastrous failure occurring primarily along certain weak planes such as grain boundaries. Understanding what causes ductile and brittle fractures is necessary to engineer materials to withstand specific loads.
Mechanisms of ductile fracture
Generally, the mechanisms of ductile fracture pass through three stages: nucleation, growth and coalescence of micro-voids. The first step is characterized by formation of microscopic voids around inclusions or second-phase particles within the material. These voids begin to grow and elongate once stress is still being exerted on them. This process is supported by plastic deformation of the matrix surrounding it. At last, they merge with adjacent voids which are expanding resulting into coalescence. These create micro-cracks that propagate throughout a material until there is a development for a macroscopic crack at some point causing final failure. Ductile cracks undergo large amounts of plastic deformation and have rough fibrous fracture surface characteristics. To predict material failures as well as improve designs and manufacturing processes for parts that experience high stresses, it is important to understand these mechanisms.
Mechanisms of brittle fracture
Brittle fracture is a fast crack propagation without any plastic strain. In sharp contrast to this, ductile fracture occurs gradually and involves a significant amount of prior plastic deformation which makes it easier to predict. The key to understanding brittle fracture rests on the concept of stress concentration; in which applied stress becomes much more intense at discontinuities like notches, holes or sharp corners. Such concentrated stresses initiate cracks under applied loading that grow rapidly.
Breakage in brittle materials normally happens under low temperature conditions and high mechanical strain rates. For instance, the shiny, granular appearance of fracture surfaces in brittle materials indicates that there has been rapid crack propagation through crystal structures within them. At the micro level, this type of fracture may follow cleavage planes in crystal lattice thus resulting into intergranular or transgranular paths of fractured crystals. Brittleness can be further worsened by such factors as material flaws, manufacturing defects and environmental conditions such as low temperatures or corrosive environments. One needs to understand brittle failure well if he/she wants catastrophic failure prevention regarding engineering applications, especially for critical infrastructure and safety-related components.
Impact of plastic deformation
Plastic deformation is the name given to the irreversible shape change that happens when stresses go beyond a material’s elastic limit. It is important in many engineering applications as it affects directly the toughness and ductility of materials. When a material undergoes plastic deformation, energy is absorbed and redistributed, which can help to blunt and arrest the growth of cracks, reducing the likelihood of sudden failure. Factors influencing plastic deformation include composition of material, temperature effects, strain rate factors and presence of impurities or defects. Understanding how materials behave under plastic deformation enables creation of components designed to withstand high-stress conditions and enhance overall structural integrity.
Why are some materials ductile and others brittle?
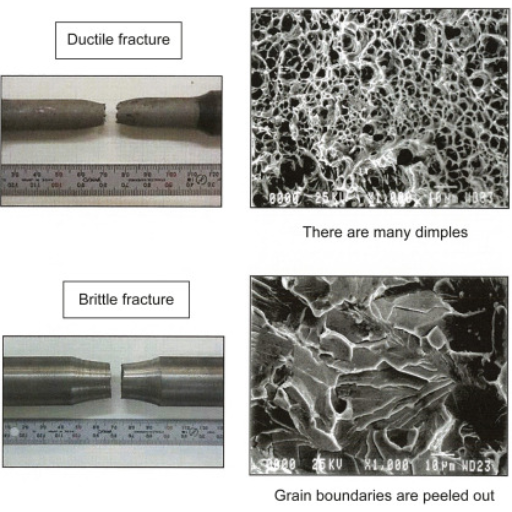
Ductile and brittle materials differ primarily in terms of their atomic structure and bonding types. Metals are examples of ductile substances because they possess an atomic structure that is flexible, meaning their bonds are capable of sliding and reshuffling during the application of tension without breaking them. This allows them to undergo plastic deformation as well absorb large energy before fracture occurs. Conversely, ceramics and glass are brittle materials with strong but rigid bonds that resist rearrangement under shear stress resulting in sudden cracking. Moreover, factors such as temperature, strain rate and presence of impurities can determine whether a material behaves in a ductile or brittle fashion. Understanding these properties leads to appropriate choice of materials for different engineering applications so that they perform satisfactorily under varied conditions.
Influence of material composition
One thing that determines whether a material is ductile or brittle to a great extent, is its composition. Mechanics can be influenced by the atomic structure as well as bonding characteristics and microstructure of elements and compounds in a material. For example, metals that are made up of atoms forming metallic bonds with free electrons have high ductility due to their ability to easily displace and reorient whenever stressed. On the other hand, ceramics and glasses with ionic or covalent bonds are less pliable and more likely to fail in a brittle manner because stronger bonds do not allow atom rearrangement. Besides, alloying additions, impurities and characteristics such as grain size control or phase distribution can also change mechanical behavior of materials from being ductile to brittle. It is necessary for the development of tailor-made engineering materials to understand these compositional influences.
The role of tensile stress
The determination of the mechanical behavior of materials under load is highly dependent on tensile stress. When tensional stress is applied to a material, it stretches and seeks to elongate. The ductility or brittleness of the material can be revealed by the ability of the material to react to such stress. Highly ductile materials tend to deform significantly before they fail by undergoing plastic deformation which results in energy absorption. In these metals, this occurrence takes place as atoms’ bonding can reform without breaking apart thus allowing for change in shape. On the other hand, brittle substances such as certain polymers and ceramics have no capacity for this bending and instead fracture when subjected to small forces. In fields like civil and mechanical engineering, an understanding about how different materials react under tensile stress is important where strength and durability are key components for safety or functionality considerations.
Environmental factors affecting fracture
Materials may be greatly affected in their fracture behavior by environmental factors. A principal factor is temperature, with high and low temperatures capable of bringing about changes in the ductility and brittleness of materials. For example, at lower temperatures metals can become more brittle, thus making them to easily fail under stress conditions. Also, high temperatures decrease strength and increase chances of deformation.
Another vital environmental factor is the presence of corroding elements such as moisture, chemicals and salts. They lead to stress corrosion cracking, which occurs when a combination of tensile stress and corrosive environment results in cracking followed by failure. This happens frequently in stainless steel and aluminum alloys.
Further, there are certain polymers that get degraded over time due to exposure to ultraviolet (UV) radiation causing brittleness. Such degradation is particularly common in outdoor applications.
In summary, one must comprehend how environmental factors affect material performance; otherwise materials used for various engineering purposes might not last or work properly.
What are the practical applications of ductile and brittle materials?

There are specific characteristics that make ductile and brittle materials useful in different practical applications. Ductile materials, for instance copper and steel, are known to undergo plastic deformation before breaking such as that they have high toughness as well as flexibility required in many applications. This includes use of these materials extensively in beams, bridges and pipelines like for electrical wiring, automotive components.
On the other hand, brittle ceramics as well as glass can hardly deform prior to fracturing; this makes them suitable for rigidly hard needs. Such materials are typically used in protective coatings, cutting tools, electronic components like insulators and substrates. It is thus true to say that both kinds of fabrics play significant functions within different engineering fields on the basis of what a particular application might require.
Common uses of ductile materials
Ductile materials are prized in many industries because they can be greatly deformed before cracking. For instance:
- Construction Industry: The construction industry relies on ductile materials like steel to construct buildings, bridges and other infrastructure. Such structures not only possess toughness and flexibility but also are capable of withstanding various loads and stresses.
- Automotive Industry: Car bodies, engine components, and chassis are created by the use of ductile materials such as steel or aluminum in automotive manufacturing. The integrity of the car is aided by these metals when they absorb energy during crash collisions thereby improving passenger safety.
- Electrical Wiring and Electronics: Copper and aluminum, which are also ductile materials, are frequently used for electrical wiring because they have good conductivities as well as being flexible. Additionally, electronic circuits and components often contain these substances thus enabling reliable electrical connections and signal integrity.
When to use brittle materials
Brittle materials are best suited for use in situations requiring high hardness, resistance to wear and ability to withstand high temperatures. These materials, which are typically not significantly deformed before breaking, perform well in environments that require them to sustain uniform behavior under crushing loads. Some common examples include:
- Cutting Tools: The brittleness of ceramics and carbides makes them indispensable as cutting tools due to their extreme hardness and wear resistant properties; they maintain sharpness and achieve accurate cuts during machining.
- Protective Coatings: In industry machineries, brittle materials such as glass have a great usage because of their high degree of hardness and resistance towards abrasion; this makes them ideal for protective coatings that can make the machine serve for long with minimum maintenance needs.
- Electronic Components: Silicon and glass are used as insulators and substrates in semiconductors and other devices in the electronics industry. Their brittleness confers the necessary stability that allows precision electronic applications.
Engineers can improve the performance, longevity, and efficiency of various products and systems by taking advantage of the attributes of brittle materials.
Balancing ductility and brittleness in engineering
Balancing engineering that is brittle and ductile means finding materials that are suitable for particular applications, while exploiting the strengths of each property. In order to achieve this balance, engineers often mix different materials in composites, apply heat treatments or use alloying to improve performance under various conditions. The following strategies are used:
- Composite Materials: A combination of ductile and brittle materials can form composites, which exhibit a synergistic effect in terms of toughness and hardness. Fiberglass is an example of a material with such properties as it combines resin’s malleability with glass’ fragility so that it becomes rigid as well as supple.
- Heat Treatments: These processes such as tempering or quenching can change the internal structure of metals thus enhancing their mechanical attributes. Heat treatment can enhance ductility in brittle metals or hardness in the case of less malleable ones depending on what is required.
- Alloying: Blending together different metals may lead to an alloy having both brittleness and ductility. For instance, by adding minute amounts of carbon to iron one gets steel which becomes stronger and more flexible compared to pure iron alone.
Engineers can select and fabricate materials so that they have designed components with specific features responding exactly to their given tasks; therefore ensuring excellent performance, security aspects and long life spans for them.
Frequently Asked Questions (FAQs)
Q: What is the main difference between brittle and ductile materials?
A: The main difference between brittle and ductile materials lies in their ability to deform before fracturing. Ductile materials can undergo significant plastic deformation before failure, while brittle materials typically exhibit minimal plastic deformation and fracture suddenly.
Q: Can you provide examples of ductile materials?
A: Examples of ductile materials include metals like copper, aluminum, and steel. These materials can be stretched into wires or bent into different shapes without breaking.
Q: What are some common examples of brittle materials?
A: Examples of brittle materials include ceramics, glass, and some cast irons. These materials tend to fail suddenly under stress without significant deformation.
Q: How do brittle and ductile fractures differ in appearance?
A: Ductile fractures generally show a rough, fibrous fracture surface due to the significant plastic deformation. On the other hand, brittle fractures usually have a smooth, flat fracture surface because of the sudden breakage with little deformation.
Q: What contributes to the brittleness and ductility of a material?
A: The brittleness and ductility of a material are influenced by factors like temperature, strain rate, and the material’s composition and microstructure. Materials typically become more brittle at lower temperatures and higher strain rates.
Q: Is there a test to determine if a material is ductile or brittle?
A: Yes, one common test is the Charpy impact test, which measures the energy absorbed by a material during fracture. High energy absorption indicates ductility, while low energy absorption indicates brittleness.
Q: Why is it important to understand ductile vs brittle behavior in materials?
A: Understanding ductile vs brittle behavior is crucial in engineering and design because it helps in selecting the right materials for specific applications, ensuring safety, reliability, and performance of structures and components.
Q: How does temperature affect whether a material is brittle or ductile?
A: Temperature greatly affects brittleness and ductility. Generally, materials tend to become more brittle at lower temperatures and more ductile at higher temperatures. This shift can have significant implications for the material’s performance in different environments.
Q: What type of materials usually have high ductility?
A: Materials that have high ductility are typically metals such as gold, silver, copper, and aluminum. These materials can endure significant plastic deformation before breaking.
Q: How do brittle materials behave under stress?
A: Brittle materials tend to fail suddenly and without significant deformation when subjected to stress. This type of material behavior is characterized by a rapid propagation of cracks leading to fracture.





